CHAPTER 2. ALTERNATIVES
This chapter identifies the alternatives that DOE has evaluated for each material type and identifies DOE's preferred alternatives. Table 2-1 lists the alternatives. Although most of the alternatives evaluated in this EIS would rely on the use of existing facilities at the SRS, some would require new or modified facilities. This chapter identifies such facilities for each alternative, if applicable. Appendix C contains detailed descriptions of the facilities and their operations.
DOE has identified three broad categories of materials (i.e., Stable, Programmatic, and Candidate for Stabilization). In general, DOE proposes to maintain Stable material in its current form, convert Programmatic material to a safe and storable form to meet future needs, and stabilize material that presents a safety concern if storage in its existing form continues. A number of steps (i.e., phases) are associated with the implementation of any alternative (other than the No-Action Alternative). The description of each alternative in this chapter includes a chart that shows the sequence and approximate duration of the steps needed to implement it; the heavier line on each chart indicates the critical time path for that alternative.
2.1 Stable Material
DOE has determined that the condition of most nuclear material at the SRS is not likely to present a safety concern over the next 10 years and that such material is stable and suitable for continued storage. Table A-1 lists each Stable material and specifies the facility in which DOE has stored it.
Because Stable material is suitable for continued storage, no actions are necessary to meet the purpose and need for this EIS. Therefore, the preferred alternative for Stable material is Continuing Storage (No Action). Under this alternative, such material would be managed in its existing form to maintain the health and safety of workers and the public.
DOE would maintain facilities in good working condition and would continue to provide utilities (water, electricity, steam, compressed gas, etc.) and services (security, maintenance, fire protection, etc.) for each facility. Training activities would ensure that appropriate personnel maintained the skills necessary to operate the facilities and equipment.
DOE would relocate, repackage, or recan the material as necessary to maintain safety. Relocation would include the movement of material to consolidate storage, allow maintenance, or respond to a safety concern. Repackaging would include placing material from a damaged storage container in a new container or placing the damaged container in a larger container. DOE could perform repackaging before damage to a container occurred if analyses concluded that damage was likely. Recanning, which would primarily involve fuel and targets, would entail placing damaged or degraded fuel in metal containers, sealing the containers, and placing them in storage. Sampling, destructive and nondestructive examination, weighing, visual inspections, and similar activities would determine the physical and chemical condition of the material. Existing solutions would require chemical adjustments to maintain their required concentration limits and chemistry controls. In addition, DOE would continue ongoing programs for the consolidation of highly enriched uranium, including the recasting of uranium fuel into ingots.
2.2 Programmatic Material
DOE has determined that some of the nuclear material at the SRS is needed to meet current or future program missions. The following paragraphs indicate the missions for such materials, which Appendix A describes in more detail:
- Plutonium-242 (Pu-242), which DOE would use in the nuclear weapons stockpile stewardship program. This program assures the safety and reliability of the existing nuclear weapons stockpile and Pu-242 is an essential and increasingly important part of the stockpile stewardship program. DOE has placed the information on the use and need for Pu-242, which is classified, in Appendix B. This appendix is available for review by the DOE decisionmaker.
- Americium-243 and curium-244, which DOE would maintain as a national asset to support research in nuclear medicine, nuclear chemistry, solid-state chemistry, and nuclear physics.
- Neptunium-237, which DOE would use in the production of plutonium-238 to provide a power source for remote terrestrial and space applications.
None of the programmatic material is in a form that DOE could use to meet its program missions. As a result, DOE has evaluated an alternative(s) for each material that would convert it to a stable and storable form for future use in DOE programs.
Almost all of the programmatic material exists in solution form (see Table 1-1). The plutonium-242, americium-243 and curium-244, and neptunium-237 solutions at the Savannah River Site present the same environmental, safety and health concerns as the Site's other plutonium solutions; however, due to the quantity of plutonium-242, and americium-243/curium-244 isotopes stored in solution, they do not present a criticality hazard. Therefore, there is a need to stabilize these solutions independent of the program need. Future DOE decisions will determine if these materials will actually be used. The Record of Decision following the completion of the Interim Management of Nuclear Materials EIS will only determine what, if any, stabilization actions will be taken for these special materials.
2.2.1 PLUTONIUM-242
The SRS plutonium-242 that could be used to meet programmatic needs is stored in an aqueous solution in one tank in H-Canyon. DOE has evaluated the following alternatives for the conversion of this plutonium-242 to a form that meets the programmatic need:
· Processing to Oxide.
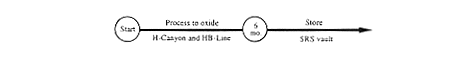
DOE would convert existing forms of plutonium-242 to an oxide by operating H-Canyon and HB-Line (Figure 2-1 shows key facilities within H-Area, including the H-Can
yon building in the center; the figure also shows the Defense Waste Processing Facility in the adjoining S-Area). Chemical separation activities would be conducted in the canyon as necessary to separate the plutonium-242 from impurities and radioactive decay products in the solution to prepare the material for conversion to a solid in HB-Line. Separated material other than plutonium-242 would be transferred from H-Canyon to the high-level waste tanks via underground pipes. The entire inventory of plutonium-242 solution in H-Canyon would be transferred through pipes to HB-Line where it would be converted to an oxide. The oxide would be packaged in steel containers and stored in an SRS vault. The material would be monitored and inspected during this storage period but the containers would be opened only to satisfy a concern about safety, material accountability, etc. When the proposed oxide packaging capability in FB-Line or the proposed Actinide Packaging Facility became available (see Appendix C), the existing inventory of material would be evaluated to determine if any action was required to ensure that the material met the DOE standard for storage of plutonium oxides (DOE 1994a). If actions were required, the material would be transferred to the packaging facility, heated, and repackaged.
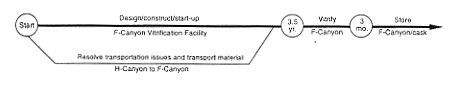
Vitrification (F-Canyon).
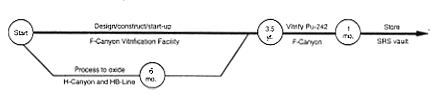
DOE would modify a portion of F-Canyon to add a vitrification capability. DOE would create the vitrification facility by modifying an area inside the hot canyon
(see Appendix C). This modified area - the F-Canyon Vitrification Facility - would take about 3-1/2 years to complete. Most of the waste generated from the modification operations would be low-level radioactive waste, which DOE would dispose of in existing SRS disposal facilities. After the facility became operational, DOE would transfer oxide from H-Canyon (produced as described above for the Processing to Oxide Alternative) to F-Area and vitrify it in the F-Canyon Vitrification Facility. DOE would store the canisters in F-Canyon or a shielded vault. As a variation, DOE could transfer the plutonium-242 solutions to F-Area using an appropriate shipping container (truck or rail). At present, however, DOE does not have the capability to make such transfers. The issues of container certification and availability must be resolved. In F-Area, the material could be moved into F-Canyon by using a transfer line in the F-Area Outside Facilities east of the canyon or by bringing the shipping container into the canyon and transferring the solution or targets to process vessels. Other transfer methods could be utilized, such as introducing the material through FB-Line. When the material was in the facility, it would be processed by chemical separation, if required, to ensure the purity of the plutonium-242. The material would be chemically adjusted as required to meet the specifications for introducing the plutonium to the vitrification process. The material would be directed through intrafacility piping to the vitrification facility where the plutonium would be combined with molten glass, poured into steel containers, cooled, and placed in storage in the canyon or a shielded vault. High-level waste generated during these operations would be transferred to the F-Area high-level waste tanks.
Processing and Storage for Vitrification in Defense Waste Processing Facility.
DOE would continue to store the plutonium-242 solutions until the completion of technical feasibility studies. These studies would be necessary to determine the potential magnitude of the plutonium-242 contribution to saltstone radioactivity and assess whether the resulting saltstone radioactivity would exceed permitted limits. When the studies were complete, DOE would adjust the solution chemically as necessary for discharge to the waste tanks and eventually vitrify the material at the proposed Defense Waste Processing Facility.
· Continuing Storage (No Action). DOE would continue to store the plutonium-242 solution in the H-Canyon tank. The activities discussed for stable material (Section 2.1) would be applicable.
DOE has identified Processing to Oxide as the preferred alternative because the SRS currently has the capability to convert the material to an oxide, and because the oxide form would meet the programmatic need. DOE reviewed conversion of the material to metal but determined it to be unreasonable for detailed analysis in the EIS. Converting the material to a metal would still require either the production of an oxide in HB-Line and then the additional steps of transferring the material to FB-Line where it would be redissolved and converted to a metal, or the transportation of liquid plutonium-242 to FB-Line. DOE determined that producing an oxide and then dissolving it to produce metal would add unwarranted environmental impacts because the oxide form would meet the programmatic need. DOE did not select transferring the plutonium-242 solution to FB-Line for conversion to metal because DOE has not developed a method to hold the plutonium-242 during transportation. DOE evaluated but did not select the Processing and Storage for Vitrification (Defense Waste Processing Facility) alternative because implementing this alternative would make the material unavailable to meet the programmatic need. The material would not be available because once it was discarded to the high-level waste tanks, it would be mixed with all other waste and diluted to the point that it would be unrecoverable. DOE evaluated but did not select the Vitrification
(F-Canyon) alternative because of the additional steps required to convert vitrified plutonium-242 to a form usable to meet the programmatic need. To make the plutonium-242 usable after vitrification, DOE would have to chemically dissolve the glass, separate the plutonium, and convert the plutonium solution to an oxide or metal.
2.2.2 AMERICIUM AND CURIUM
About 14,000 liters (3,800 gallons) of americium and curium solution are stored in a single tank in F-Canyon (Figure 2-2 shows F-Area with the F-Canyon building in the center). Americium and curium are feed materials in the DOE National Heavy Metal and Advanced Neutron Source Program that produces heavier transuranium elements such as californium-252. Californium-252 has a wide variety of medical, commercial, and defense-related uses, which include cancer treatment and treatment research, neutron radiography for nondestructive testing of metal parts in aircraft, and the online assay of coal and cement as a quality control function.
DOE has determined that to be suitable for eventual programmatic use the material should be converted to a solid form that could be transported to and used by the Oak Ridge National Laboratory (the DOE user). DOE would have to convert the americium and curium solution in F-Canyon to a solid to meet these programmatic uses.
DOE has identified the following alternatives for evaluation in considering conversion of the americium and curium material to meet programmatic needs:
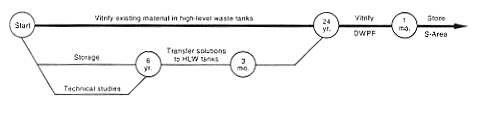
· Vitrification (F-Canyon).
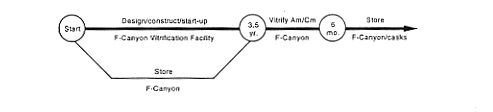
DOE would continue to store the material in F-Canyon while undertaking studies, design work, and modification of a portion of the canyon to add a vitrification capabi create the vitrification facility by modifying an area inside the hot canyon (see Appendix C). This modified area - the F-Canyon Vitrification Facility - would take about 3-1/2 years to complete. Most of the waste generated from the modification operations would be low-level radioactive waste, which DOE would dispose of in existing SRS disposal facilities.
Figure 2-2. F-Area.
After the facility became operational, DOE would process the existing americium and curium solution to remove impurities and radioactive decay products and chemically adjust the material as necessary to meet vitrification process feed requirements. Then the material would be transferred to the vitrification facility. DOE would vitrify the material, pour it into stainless-steel canisters, seal the canisters, and place them in storage at the SRS. DOE expects it would take about 6 months to vitrify the americium and curium solutions, producing about 40 canisters. The radiation level would be very high, about 90 rem per hour at 1 meter (3.2 feet) from a canister. High-level waste generated from chemical processing operations would be transferred to the F-Area high-level waste tanks.
· Processing to Oxide.
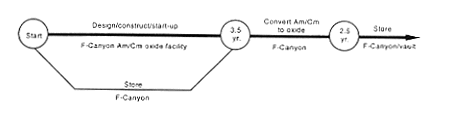
DOE would continue to store the material in F-Canyon while undertaking studies, design work, and modification of a portion of the canyon to add the capa
bility to process americium and curium to oxide. These modifications would take about 3-1/2 years to complete. A problem associated with oxide production is that the operation of the process would be limited to batches of 500 grams (17.6 ounces). Larger quantities would cause self-heating of the material to an extent that would impede the oxide conversion process. At this rate, it would take about 2-1/2 years to convert all the americium and curium to oxide even if DOE operated the conversion facility 24 hours a day, 7 days a week. This operation would yield about 250 cans of americium and curium oxide. Another problem is that the americium and curium oxide would emit very high levels of radiation. Each can of oxide could produce radiation levels as high as 30 rem per hour at 1 meter (3.2 feet). As a result, all loading and packaging operations (which are normally performed by hand) would have to be accomplished remotely. Designs for this remote operation would be complicated and would be the factor of greatest uncertainty associated with the implementation of this alternative. In addition, DOE has not yet been able to identify a suitable container (the cask into which it could load the oxide cans) for storage and eventual shipment.
After the facility became operational, DOE would process the existing americium and curium solution to remove impurities and radioactive decay products and chemically adjust the material as necessary to meet the oxide conversion process feed requirements. Then the solution would be transferred through pipes inside the canyon to the oxidation facility. The material would be converted to an oxide, sealed in containers, and placed in appropriate storage canisters. The material would be stored in F-Canyon or transferred to a heavily shielded vault for storage. High-level waste generated during processing would be sent to the F-Area high-level waste tanks via underground pipes.
Processing and Storage for Vitrification in Defense Waste Processing Facility.
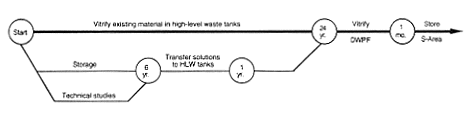
DOE would continue to store the americium and curium solutions until the completion of technical feasibility studies. These studies would be necessary to determine
the potential magnitude of the americium and curium contribution to saltstone radioactivity and assess whether the resulting saltstone radioactivity would exceed permitted limits. When the studies were complete, DOE would adjust the resulting solution chemically as necessary for discharge to the waste tanks and eventually vitrify the material at the proposed Defense Waste Processing Facility.
· Continuing Storage (No Action). DOE would continue to store the americium and curium solution in F-Canyon. The activities discussed for stable material (Section 2.1) would be applicable.
DOE has identified Vitrification (F-Canyon) as the preferred alternative to convert the americium and curium solution. The construction of facilities for vitrification and oxide production would have roughly the same cost and would require the same time for completion. The vitrified material, however, would be more stable, less dispersible, and less leachable than oxide. The vitrification process would also produce fewer containers, which would be more suitable for transportation and storage, than the oxide process. DOE also expects container loading and handling procedures for the vitrified material to be less complex than those for oxide. Finally, DOE would complete the vitrification alternative about 2 years before the oxide alternative due to the operational limitations associated with oxide production.
DOE evaluated but did not select the Processing and Storage for Vitrification (Defense Waste Processing Facility) alternative because implementing this alternative would make the material unavailable to meet the programmatic need. The material would not be available because once it was discarded to the high-level waste tanks, it would be mixed with all other waste and diluted to the point that it would be unrecoverable. In addition, the increased radiation levels expected to be generated by introducing this material to the high-level waste tanks could be reduced only by diluting the waste volume with an additional one million gallons of liquid waste.
2.2.3 NEPTUNIUM-237
Approximately 6,100 liters (1,600 gallons) of neptunium-237 solution are currently stored in H-Canyon storage tanks. In addition, nine neptunium targets are stored in M-Area. Neptunium-237 is used in the production of plutonium-238, the principal use of which is in thermal power generators in applications where solar power or chemical batteries are not practical, such as exploratory spacecraft. DOE has identified the following alternatives for evaluation in considering conversion of the neptunium-237 in targets and solution to a form that could be used to meet programmatic needs:
· Processing to Oxide.
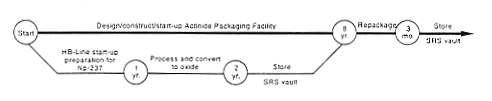
DOE would begin by transferring the nine targets from M-Area to H-Canyon and dissolving them. This material would be processed through the canyon and added to th
e existing neptunium solution. DOE would perform chemical separation operations as required to remove radioactive decay products and other chemicals that could interfere with the oxide conversion process. The resulting neptunium solution would be transferred to the HB-Line through intrafacility pipes and converted to neptunium oxide. The radioactive decay products and other material would be transferred through underground pipes to the high-level waste tanks. The oxide would be put in shielded containers and placed in storage in an F-Area vault. When the proposed Actinide Packaging Facility became available or the proposed FB-Line modifications for oxide packaging were completed (see Appendix C), any material that had not been used for programmatic purposes would be heated and repackaged if required to ensure long-term stability.
· Vitrification (F-Canyon).
DOE would continue to store the material in H-Canyon. During this time, DOE would complete the necessary technical evaluation to determine the feasibility of o
btaining a container that would enable the shipment of neptunium solutions across the SRS. In addition, DOE would undertake the studies, design work, and required equipment changes to provide the capability to vitrify neptunium-237 in F-Canyon (see Appendix C). Then DOE would transfer the neptunium-237 targets and solution to F-Canyon or FB-Line, using an appropriate shipping container (truck or rail). At present, DOE does not have the capability to make such transfers. The issues of container certification and availability must be resolved. In F-Area, the material could be moved into F-Canyon by using a transfer line in the F-Area Outside Facilities east of the canyon or by bringing the shipping container into the canyon and transferring the solution or targets to process vessels. Other transfer methods could be utilized, such as introducing the material through FB-Line. When the material was in the facility, it would be processed by chemical separation, if required, to ensure the purity of the neptunium-237. The material would be chemically adjusted as required to meet the specifications for introducing the neptunium to the vitrification process. The material would be directed through intrafacility piping to the vitrification facility where the neptunium would be combined with molten glass, poured into steel containers, cooled, and placed in storage in the canyon or a shielded vault. High-level waste generated during these operations would be transferred to the F-Area high-level waste tanks.
Processing and Storage for Vitrification in Defense Waste Processing Facility.
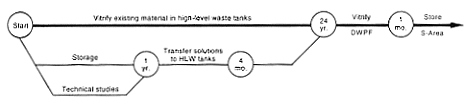
DOE would continue to store the neptunium solutions until the completion of technical feasibility studies. These studies would be necessary to determine the pote
ntial magnitude of the neptunium contribution to saltstone radioactivity and assess whether the resulting saltstone radioactivity would exceed permitted limits. When the studies were complete, DOE would adjust the resulting solution chemically as necessary for discharge to the waste tanks and eventually vitrify the material at the proposed Defense Waste Processing Facility.
· Continuing Storage (No Action). DOE would continue to store the neptunium solution in H-Canyon and the targets in M-Area or another suitable storage facility on the Site. The no-action activities discussed for stable material (Section 2.1) would be applicable for the neptunium.
DOE has determined that the preferred alternative for neptunium-237 is Processing to Oxide because the existing technology for the production of plutonium-238 is based on the use of neptunium-237 targets, which use neptunium oxide as a raw material. Although the targets in M-Area are in an oxide form, they were fabricated originally to be irradiated in the SRS reactors and cannot be used anywhere else in their current form. The SRS reactors are no longer operating. Processing the targets would place the material in a form such that future users of the material (e.g., Oak Ridge National Laboratory) could fabricate the type of target required for their plutonium-238 production process. The Processing to Oxide Alternative would use existing SRS capabilities to produce a product that met programmatic needs. The Vitrification (F-Canyon) alternative was not selected because of unresolved technical issues concerning the shipment of neptunium in liquid form and because dissolution and chemical recovery operations would be required after vitrification to enable the use of the material to fabricate targets. DOE evaluated but did not select the Processing and Storage for Vitrification (Defense Waste Processing Facility) alternative because implementing this alternative would make the material unavailable to meet the programmatic need. The material would not be available because once it was discarded to the high-level waste tanks, it would be mixed with all other waste and diluted to the point that it would be unrecoverable.
2.3 Candidate Materials for Stabilization
DOE would stabilize a material if its physical form or storage configuration was a safety concern, or if it could become a safety concern within the next 10 years. DOE evaluated a range of alternative stabilization methods for each category of nuclear material, and used the following criteria to select the alternative stabilization methods for evaluation:
- The product of the proposed action should be stable over a reasonable period of time to prevent the need to restabilize the material.
- The stabilization method should involve a technology that would enable the initiation of stabilization actions as quickly as practical and within the period covered by this EIS.
After applying these criteria, DOE selected Processing to Metal, Processing to Oxide, Blending Down to Low Enriched Uranium, Processing and Storage for Vitrification (Defense Waste Processing Facility), Vitrification (F-Canyon), and Improving Storage as reasonable alternative stabilization methods for evaluation in addition to the No-Action Alternative.
DOE has identified a preferred alternative to stabilize the material in each category. Sections 2.4 and 2.5 summarize the results of the DOE evaluation, which concluded there were no significant differences in environmental impacts among the alternatives. DOE selected the preferred alternative in each material category that would achieve stabilization quickly, emphasizing the use of proven technology and existing facilities.
2.3.1 H-CANYON PLUTONIUM-239 SOLUTIONS
Approximately 34,000 liters (9,000 gallons) of plutonium nitrate solutions are stored in stainless-steel tanks in the H-Canyon facility. DOE has identified the following alternatives for management of these solutions:
· Processing to Oxide.
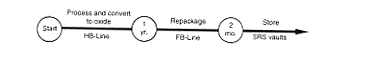
DOE would process the plutonium-239 solution by operating H-Canyon as necessary to remove radioactive decay products and other impurities that would interfere with subsequent stabilization steps. No actions would occur to achieve a specific purity for the plutonium in the solution other than those necessary to operate the process. DOE would transfer the separated impurities to the H-Area high-level waste tanks, and would transfer the plutonium solution to the HB-Line for conversion to an oxide. DOE would place the oxide in storage containers, load the containers in shipping containers, and transport the material to F-Area for storage. In parallel with this effort, DOE would modify a portion of the existing FB-Line to provide the capability to package plutonium oxide in a manner that met the storage criteria the Department has established for plutonium oxides (DOE 1994a). A glovebox would be added to FB-Line to enable the oxide to be heated and packaged in a nonreactive atmosphere without the use of plastic wrapping material. After the completion of the FB-Line modifications, DOE would transfer the plutonium oxide to that facility, heat it to meet long-term storage criteria, package it, and transfer it to a storage vault in F-Area.
If it determined that it could not modify the FB-Line to provide the proper packaging capability or the capability for future inspection and packaging maintenance, DOE would begin work on the proposed Actinide Packaging Facility (see Appendix C); this would occur in parallel with plutonium conversion activities, but the facility would take about 8 years to complete and begin operations.
The Actinide Packaging Facility or the modifications to FB-Line would provide the capability to package plutonium oxide (or plutonium metal) to meet recent Departmental recommendations for the safe storage of plutonium metal and oxides (DOE 1994a). For plutonium oxides, the recommended packaging criterion is that the material be heated to achieve a condition where less than 0.5 percent of the weight of the material is lost by subsequent heating (over a specified time period) and that, following the heating step, the material is cooled and packaged for storage in a nonreactive atmosphere so the benefits of the heating step are retained. The purpose of these actions is to minimize the amount of gas generated within the container used to store the material because the gas has the potential to pressurize, and occasionally cause failure of, a storage container. Gas, normally oxygen and hydrogen, could be generated from the decomposition of water molecules by the radiation given off by the plutonium. The new heating and packaging steps would substantially reduce the amount of moisture in the plutonium oxide, thus reducing potential gas generation. For metal, the criterion is to package the material in a nonreactive atmosphere with no contaminants such as plastic wrapping. The existing B-Line facilities at the SRS (where packaging traditionally occurred) do not have the equipment required to accomplish these new steps.
· Processing and Storage for Vitrification (Defense Waste Processing Facility).
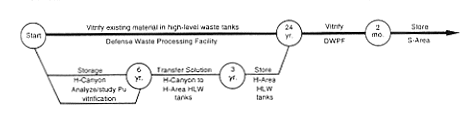
DOE would continue to store the H-Canyon plutonium solution until ready to discharge it to the H-Area high-level waste tanks. The material would even
tually be vitrified at the proposed Defense Waste Processing Facility (DWPF).
The DWPF was designed to process 132.5 million liters (35 million gallons) of high-level waste (currently stored in F- and H-Area waste tanks) into a glass material encased in stainless-steel cylinders that would be suitable for disposal in a geologic repository. The first step for vitrifying the H-Canyon plutonium solutions would be to transfer the solutions to the high-level waste tanks, which will feed the DWPF. Before transfer, DOE would adjust the solutions to ensure the nuclear criticality safety of the material in the tanks. DOE has identified several concepts for such adjustments: diluting the solutions with water and chemicals to achieve very low fissile material concentrations, diluting the solutions with depleted uranium, or adding iron and manganese or other neutron poisons such as gadolinium (DOE 1994b). After transfer to the waste tanks, the material would be stored and eventually transferred to the DWPF for vitrification.
DOE would have to address many technical issues to demonstrate the feasibility of this stabilization method. For example, a detailed safety analysis would be performed to evaluate and develop controls to prevent an inadvertent nuclear criticality accident. This type of accident could occur if the fissile material, without adequate neutron poison, precipitated during or after the transfer to the waste tanks. A complete evaluation of the capability of the proposed Defense Waste Processing Facility to process fissile material-bearing high-level waste would be required because the original vitrification process was not designed to handle significant quantities of fissile material. In addition, DOE would have to review the availability of sufficient space in the waste tanks and incorporate impacts into established plans and schedules for consolidating and processing the material in the tanks and retiring older tanks from service. Because of these complex issues, DOE estimates it would need approximately 6 years to perform the technical studies, training, and qualification efforts necessary to ensure safe operations for the transfer of the solution for subsequent vitrification. Then DOE would need 3 years or more to transfer the solutions to the high-level waste tanks because of the availability of tank space and nuclear criticality concerns. The actual vitrification of fissile material solutions in the DWPF would not start within the 10-year period evaluated in this EIS. However, the annual impacts from the work associated with the vitrification process are presented in Appendix D.
· Vitrification (F-Canyon).
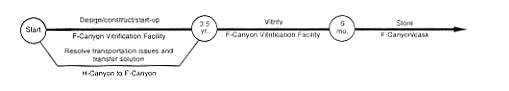
DOE would complete the necessary technical evaluation to determine if it would be feasible to obtain a container suitable to enable the shipment of plutonium solutions across the SRS. At present, DOE does not have the capability to make such transfers. The issues of container certification and availability must be resolved. In addition, DOE would undertake the studies, design work, and equipment changes required to provide the capability to vitrify plutonium in F-Canyon (see Appendix C). Then DOE would transfer the H-Canyon plutonium solution to F-Canyon or FB-Line, using an appropriate shipping container (truck or rail). In F-Area, the material could be moved to F-Canyon by using a transfer line in the F-Area Outside Facilities east of the canyon or by bringing the shipping container into the canyon and transferring the solution to process vessels. Other transfer methods could be used, such as introducing the material through FB-Line. When the material was in the facility, it would be processed by chemical separation and chemically adjusted as required to meet the specifications for introducing the plutonium to the vitrification process. The material would be directed through intrafacility piping to the vitrification facility where the plutonium would be combined with molten glass, poured into stainless-steel canisters, cooled, and placed in storage in the canyon or a shielded vault. High-level waste generated during these operations would be transferred to the F-Area high-level waste tanks.
· Processing to Metal.
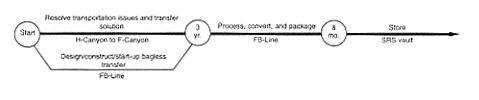
DOE would complete the necessary technical evaluation to determine the feasibility of obtaining a container that would enable the shipment of plutonium solutions across the SRS. At present, DOE does not have the capability to make such transfers. The issues of container certification and availability must be resolved. Then DOE would transfer the H-Canyon plutonium solution to F-Canyon or FB-Line, using an appropriate shipping container (truck or rail). In F-Area, the material could be moved into F-Canyon by using a transfer line in the F-Area Outside Facilities east of the canyon or by bringing the shipping container into the canyon and transferring the solution to process vessels. Other transfer methods could be used, such as introducing the material through FB-Line. When the material was in the facility it would be processed via chemical separation as required to meet the specifications for introducing the plutonium to FB-Line. No actions would occur to achieve a specific purity for this material other than those necessary to operate the process. The solution would be transferred through the FB-Line process equipment and converted to metal buttons. The buttons would be packaged and stored in an F-Area vault. Any high-level waste generated during this process would be transferred to the F-Area high-level waste tanks. In parallel with this effort, DOE would begin modifications to FB-Line to provide the capability to package plutonium metal in accordance with the Departmental plutonium storage standard (DOE 1994a). A glovebox would be added to the FB-Line facility to enable the material to be packaged in a nonreactive atmosphere without the use of plastic wrapping material. After the modifications, DOE would transfer the plutonium metal there and package it to meet DOE storage requirements for plutonium metal (i.e., the metal would be cleaned and repackaged in a nonreactive atmosphere and sealed in a container). The packaged material would be placed in an F-Area vault.
If DOE determined that it could not modify the FB-Line to provide the proper packaging capability or the capability for future inspection and packaging maintenance, DOE would begin work on the proposed Actinide Packaging Facility; this would occur in parallel with plutonium conversion activities, but the facility would take about 8 years to complete and begin operations.
· Continuing Storage (No Action). DOE would continue to store the plutonium-239 solution in H-Canyon. The no-action activities described for stable material (see Section 2.1) would be applicable for this solution.
DOE's preferred alternative is Processing to Oxide because it would rely the most on proven technology and processes and existing facilities, and because it would achieve the most important step of the stabilization process (i.e., conversion to a solid) 1 year sooner than any other alternative. The Vitrification (F-Canyon) and the Processing to Metal Alternatives were not selected because of the implementation time and unresolved technical issues associated with shipping plutonium in liquid form. DOE did not select the Processing for Storage and Vitrification (Defense Waste Processing Facility) Alternative because it could not begin the stabilization activity within the next 10 years and because of the technical uncertainties associated with processing significant quantities of fissile material through the DWPF.
DOE did not consider alternatives that would improve the methods of storing the solutions (beyond that of the No-Action Alternative) as reasonable because the material would not be in a stabilized form.
2.3.2 H-CANYON URANIUM SOLUTION
There are approximately 228,000 liters (60,000 gallons) of enriched uranium nitrate solutions in stainless-steel tanks both inside and outside the H-Canyon facility. DOE has identified the following alternatives for management of these solutions.
· Blending Down to Low-Enriched Uranium.

Before stabilizing the enriched uranium, DOE would process the solutions through H-Canyon to separate the enriched uranium from the other material in the solution (e.g., radioactive decay products normally present in irradiated fuel). The decay products would be highly radioactive and DOE would not be able to introduce it to the uranium processing equipment because of the hazard it would present to workers. DOE would transfer the radioactive decay products and other material to the H-Area high-level waste tanks. DOE would stabilize the highly enriched uranium solution (comprising approximately 60 percent uranium-235) by converting the material to uranium oxide.
The FA-Line is the only SRS facility designed to produce uranium oxide, but it was not designed to produce oxide from solutions of highly enriched uranium. To use the FA-Line, DOE would dilute the uranium-235 solution with existing depleted uranium oxide. DOE would accomplish this by dissolving the depleted uranium oxide in FA-Line. DOE would transport the depleted uranium solution to H-Canyon by truck and blend it with the enriched uranium solution to achieve a diluted solution of uranium-235. DOE would transport the mixture back to FA-Line by truck and convert it to low-enriched uranium oxide. The final product would be loaded into 208-liter (55-gallon) drums for storage. DOE would make minor modifications in F- and H-Areas to enable truck-trailer loading and unloading and to install a spare oxide dissolver at FA-Line. In addition, DOE would construct a storage facility with an area of approximately 186 square meters (2,000 square feet) on previously disturbed land in the industrialized F-Area to handle the drums of uranium oxide.
A variation of this alternative would be to transport the uranium solution from H-Area to F-Area by rail or truck using an appropriate shipping container. FA-Line would be used to dissolve depleted uranium oxide and blend it with the uranium solution from H-Area to achieve a low-enriched uranium solution. Blending operations could occur in F-Canyon process vessels or in F-Area Outside Facility tanks. The facility modifications and the storage facility described above would be required.
· Processing to Oxide (Uranium Solidification Facility).
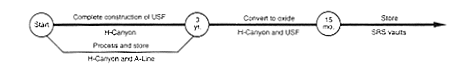
DOE would continue to store the enriched uranium solution in H-Canyon while completing construction of the Uranium Solidification Facility in the canyon. After construction, DOE would use the H-Canyon process to remove radioactive decay products and other material from the solution and would transfer the solution to the Uranium Solidification Facility using intrafacility piping. DOE would process the solution to highly enriched uranium oxide, place the oxide in containers, and store the containers in a vault.
· Processing and Storage for Vitrification in Defense Waste Processing Facility.

DOE would continue to store the H-Canyon uranium solution until it was ready for transfer to the H-Area high-level waste tanks. Before the transfer, DOE would adjust the solution to ensure the safety of the material already in the tanks. The material would be vitrified at the proposed Defense Waste Processing Facility. Criticality concerns similar to those described in Section 2.3.1 would exist for this alternative.
· Continuing Storage (No Action). DOE would continue to store the uranium solution in H-Canyon. The no-action activities described for stable material (see Section 2.1) would be applicable for this solution.
DOE's preferred alternative is Blending Down to Low-Enriched Uranium because it would achieve stabilization at least 2 years faster than any other alternative and would use existing facilities and equipment with only minor modifications. Construction of the new storage facility would not be critical to the completion of this alternative because DOE would store any drums of low enriched uranium oxide in other facilities on a temporary basis until it had completed the new storage facility. DOE did not select Processing to Oxide (Uranium Solidification Facility) because it would require the construction of a new facility, and stabilization could not occur until the completion of construction and the subsequent staffing, training, and readiness review activities. DOE did not select the Processing for Storage and Vitrification (Defense Waste Processing Facility) Alternative because it could not begin the stabilization activity within the next 10 years and because of the technical uncertainties associated with processing significant quantities of fissile material through the proposed Defense Waste Processing Facility.
DOE did not evaluate Processing to Metal in detail because this capability does not exist at the SRS (facilities would have to be modified or constructed); in addition, because the oxide form is stable, there would be no advantage to producing uranium metal. DOE did not evaluate Improving Storage because this method would be viable only for material already in solid form.
2.3.3 PLUTONIUM AND URANIUM STORED IN VAULTS
The material in this category is currently stored in about 3,000 containers, most of which are small cans in either the Building 235-F vault or the FB-Line vault. The material includes alloys, compounds, oxides, large metal pieces such as buttons and ingots, and metal fragments, and consists predominantly of plutonium-239 with some uranium-235.
DOE anticipates that the material would fall into one of two categories. The first would be material for which DOE could achieve stabilization by simply heating and repackaging to meet the long-term storage criteria (DOE 1994a). The material in this category would generally be lower in chemical contaminants and higher in the percentage of fissile material; examples include plutonium metal (such as buttons) and plutonium and uranium oxides, which are essentially in product form. The other category of material would require some type of processing action to achieve stabilization. The material in this category would be higher in chemical contaminants (such as reactive calcium and fluorides) and lower in the percent of fissile material; examples include plutonium compounds, metal fragments, and plutonium and uranium oxides that are residual material from past production activities. DOE believes about half of all the containers hold material that would require only heating and repackaging; the remaining material would require a stabilization activity that involves processing. DOE has identified Continuing Storage (No Action), Improving Storage, Processing and Storage for Vitrification in Defense Waste Processing Facility, Processing to Oxide, Processing to Metal, and Vitrification (F-Canyon) as alternatives for the management of this material.
· Improving Storage.
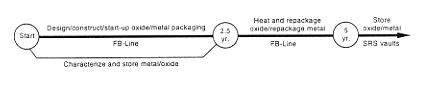
DOE would upgrade its container inspection capability by installing new equipment in an existing facility such as FB-Line; this would consist of installing digital radiography screening equipment and other assay equipment to assess the condition of the material and the containers. DOE would transfer the containers to the inspection area to determine the condition of the material. Material determined to require processing before repackaging would be returned to storage until processing activities could be initiated. Material determined to require only repackaging would be returned to storage until the repackaging facility was completed.
In parallel with these inspection activities, DOE would begin work to provide the capability to meet the Departmental plutonium storage standard (DOE 1994a) in FB-Line. A glovebox would be added to heat plutonium oxide and to package oxide and metal in a nonreactive atmosphere without the use of plastic wrapping material. After the modifications were completed, DOE would transfer the plutonium oxide there for packaging. The packaged material would be placed in a SRS vault. High-level waste from these processing operations would be sent to the F-Area high-level waste tanks.
If DOE determined that it could not modify the FB-Line to provide the proper packaging capability or the capability for future inspection and packaging maintenance, DOE would begin work on the proposed Actinide Packaging Facility. This would be accomplished in parallel with plutonium inspection and characterization activities, but the facility would take about 8 years to complete and begin operations. Any plutonium oxide that had not been packaged to meet the DOE plutonium storage criteria (DOE 1994a) would be transferred to the facility and repackaged.
· Processing to Oxide.
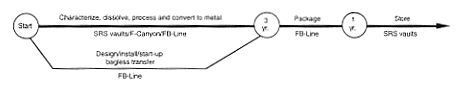
DOE would transfer potentially unstable oxide or metal from storage to HB-Line or H-Canyon. DOE would dissolve the material in one of the HB-Line or H-Canyon dissolvers and process it as required in the canyon to separate the plutonium from the uranium and other impurities that contributed to the stability concerns. The plutonium would be processed through HB-Line to produce an oxide, which would be placed in a vault for storage. No actions would occur to achieve a specific purity for this material other than those necessary to operate the process. The uranium would be diluted to low enrichment, converted to an oxide, and packaged as described for the H-Canyon Uranium Solutions (see Section 2.3.2). As a variation, the uranium could be chemically adjusted and transferred to the H-Area high-level waste tanks. The amount of fissile material involved in this transfer would be small, obviating the criticality concerns described for the Processing and Storage in the Defense Waste Processing Facility Alternative. In parallel with this effort, DOE would begin work to provide the capability to meet the Departmental plutonium storage standard (DOE 1994a) in FB-Line. A glovebox would be added or modified to heat and package the material in a nonreactive atmosphere without the use of plastic wrapping material. After the modifications, DOE would transfer the plutonium oxide there for packaging. The packaged material would be placed in an F-Area vault. High-level waste from these processing operations would be sent to the H-Area high-level waste tanks.
If DOE determined that it could not modify the FB-Line to provide the proper packaging capability or the capability for future inspection and packaging maintenance, it would begin work on the proposed Actinide Packaging Facility. This would be accomplished in parallel with oxide conversion activities, but the facility would take about 8 years to complete, and begin operations. Any plutonium oxide that had not been packaged to meet the DOE plutonium storage criteria (DOE 1994a) would be transferred to the facility and repackaged.
Processing to Oxide.
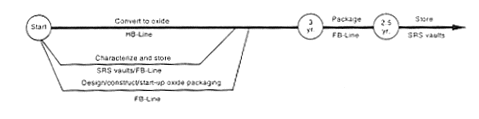
DOE would transfer potentially unstable oxide or metal from storage to F-Canyon or FB-Line, dissolve the material in one of the F-Canyon or FB-Line dissolvers, and process it as required in the canyon to separate the plutonium from the uranium and other impurities that contributed to the stability concerns. The plutonium would be processed through the FB-Line to produce plutonium metal, which would be packaged and placed in a vault for storage. No actions would occur to achieve a specific purity for this material other than those necessary to operate the process. The uranium would be processed to low enrichment by blending it with depleted uranium using FA-Line and F-Canyon process vessels or F-Area Outside Facilities tanks, as described for the H-Canyon Uranium Solutions (see Section 2.3.2). As a variation, the uranium could be chemically adjusted and transferred to the F-Area high-level waste tanks. The amount of fissile material involved in this transfer would be small, obviating the criticality concerns described for the Processing and Storage in the Defense Waste Processing Facility Alternative. In parallel with this effort, DOE would begin work to provide the capability to meet the Departmental plutonium storage standard (DOE 1994a) in FB-Line. A glovebox would be added or modified to package the material in a nonreactive atmosphere without the use of plastic wrapping material. After the modifications, DOE would transfer the plutonium metal there for packaging. The packaged material would be placed in an F-Area vault. High-level waste from these processing operations would be sent to the H-Area high-level waste tanks.
If DOE determined that it could not modify the FB-Line to provide the proper packaging capability or the capability for future inspection and packaging maintenance, it would begin work on the proposed Actinide Packaging Facility. This would be accomplished in parallel with plutonium conversion activities, but the facility would take about 8 years to complete, and begin operations. Any plutonium metal that had not been packaged to meet the DOE plutonium storage criteria (DOE 1994a) would be transferred to the facility and repackaged.
Processing and Storage for Vitrification in Defense Waste Processing Facility.
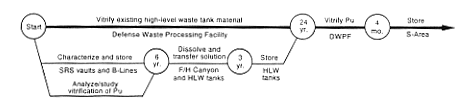
DOE would store the material until it was ready to transfer it to the F- or H-Area high-level waste tanks. In preparing the material for transfer to the waste tanks, DOE would move it to FB-Line or F-Canyon or to HB-Line or H-Canyon and dissolve it. DOE would adjust the solution to ensure the safety of the material in the waste tanks and then would transfer the material to the F- or H-Area high-level waste tanks. The material would be vitrified at the proposed Defense Waste Processing Facility. The difficulties associated with this alternative are the same as those described in Section 2.3.1
Vitrification (F-Canyon).
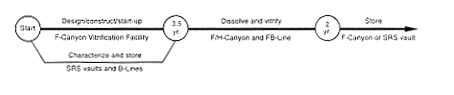
DOE would store the potentially unstable oxide and metal until the proposed F-Canyon Vitrification Facility was available. Then the material would be transferred to F-Canyon or FB-Line and dissolved and processed in the canyon to separate the plutonium and uranium and other impurities. The plutonium would be chemically adjusted as required to achieve the feed specifications for vitrification and then vitrified. The resulting glass product in stainless-steel canisters would be stored in F-Canyon or a vault. The uranium would be processed to low enrichment by blending it with depleted uranium using FA-Line and F-Canyon process vessels or F-Area Outside Facilities tanks, as described in Section 2.3.2.
As a variation, the uranium could be chemically adjusted and transferred to the F-Area high-level waste tanks. The amount of fissile material involved in this transfer would be small, obviating the criticality concerns described for the Processing and Storage in the Defense Waste Processing Facility Alternative. Any high-level waste associated with this alternative would also be sent to the F-Area high-level waste tanks.
Continuing Storage (No Action). DOE would continue to store the plutonium solids in a vault. The no-action activities described for stable material (see Section 2.1) would be applicable for these solids.
DOE proposes Improving Storage and Processing to Metal as the preferred alternatives for stabilizing this material. As mentioned above, DOE believes that about half the containers hold material for which the Improving Storage Alternative would be applicable. The material in the remaining containers would be stabilized by the Processing to Metal Alternative. DOE would use the Processing to Metal Alternative because it would achieve stabilization about 18 months sooner than Vitrification (F-Canyon) and about 2 years more quickly than Processing to Oxide. In addition, the metal alternative would rely the most on the use of existing capability and technology. The alternative of vitrification in the Defense Waste Processing Facility was not selected because stabilization activity could not be initiated within the next 10 years (or more) due to the technical issues and the inventory of existing high-level waste that would have to be vitrified first.
2.3.4 MARK-31 TARGETS
Approximately 16,000 metal targets are stored in water-filled basins in K- and L-Areas and the F-Canyon. These aluminum-clad targets contain depleted uranium, plutonium-239, and fission products. DOE has identified the following reasonable alternatives for the interim management of these targets:
Processing to Metal.
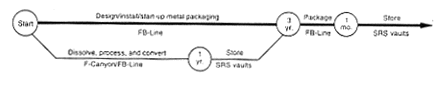
DOE would load the targets from the disassembly basins into large casks, load the casks on SRS rail cars, and transport them to F-Canyon, where it would load the targets in a dissolver tank and dissolve the targets. Then DOE would use the PUREX process to separate the plutonium solution from depleted uranium, fission products, and other impurities. DOE would process the depleted uranium to oxide in FA-Line and store it in F-Area, and would process the plutonium to metal in FB-Line. No actions would occur to achieve a specific purity for this material other than those necessary to operate the process. DOE would place the metal in containers and store the containers in a vault. In parallel with this effort, DOE would modify a portion of the existing FB-Line to provide the capability to package plutonium metal in a manner that met the storage criteria the Department has established for plutonium (DOE 1994a). A glovebox would be added to FB-Line to enable the metal to be packaged in a nonreactive atmosphere without the use of plastic wrapping material. On completing the modification to the FB-Line, DOE would repackage the material to meet the long-term storage criteria for plutonium metal.
If DOE determined that it could not modify the FB-Line to provide the proper packaging capability or the capability for future inspection and packaging maintenance, DOE would begin work on the proposed Actinide Packaging Facility; this would occur in parallel with plutonium conversion activities, but the facility would take about 8 years to complete and begin operations.
· Processing to Oxide.
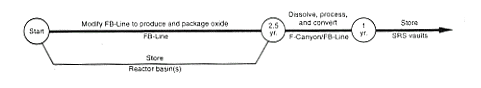
DOE would load the targets from the disassembly basins into casks, load the casks on SRS rail cars, and transport them to F-Canyon, where it would load the targets in a dissolver tank and dissolve the targets. Then DOE would use the PUREX process to separate the plutonium solution from depleted uranium, fission products, and other impurities. DOE would modify the FB-Line to support conversion of the plutonium solutions to plutonium oxide and to package the material for storage. No actions would occur to achieve a specific purity for the material other than those necessary to operate the process. DOE would produce a material form and packaging configuration that met the DOE standard for long-term storage of plutonium oxide (DOE 1994a). DOE would process the depleted uranium to an oxide in FA-Line and store the material in F-Area. Any high-level waste from these processing activities would be transferred to the F-Area high-level waste tanks.
If the extent of the FB-Line modifications necessary to meet the DOE plutonium storage standard were economically or physically impractical (i.e., too expensive or not enough space for the equipment required), the Department would perform the stabilization effort in two phases. DOE would convert the material initially to an oxide form and package it in FB-Line. In parallel, DOE would construct the proposed Actinide Packaging Facility. The oxide initially produced would be stored in a vault until the new facility was available. DOE estimates the minimum required modifications to FB-Line would take about 3 years to complete. DOE expects the Actinide Packaging Facility would be available in approximately 8 years.
DOE considered two other variations of this alternative. DOE could dissolve the Mark-31 targets in H-Canyon and process the resulting plutonium solutions into an oxide in HB-Line. This variation would require modification of the HB-Line to provide the capability to package the resulting oxide in accordance with the DOE standard for long-term storage of plutonium. Approximately 3 years would be required to make the necessary modifications. However, even if DOE modified HB-Line, the volume of depleted uranium contained in the Mark-31 targets as compared to the capacity of H-Canyon to dissolve and process, would require the operation of H-Canyon for over 30 years.
As another variation, DOE could dissolve the Mark-31 targets in F-Canyon, transport the resulting plutonium solutions to H-Canyon, and convert the plutonium to an oxide using HB-Line. Approximately 1 year would be required to modify the H-Canyon and F-Canyon facilities to provide the capability to load and unload the solutions into a transport container. DOE does not currently have a container designed to transport liquid plutonium, but is exploring the availability of such a container internationally. As in the variation described above, approximately 3 years would be required to modify HB-Line to provide the capability to package the oxide in accordance with the DOE standard. It would take over 6 years to convert the solutions to an oxide in HB-Line, as opposed to approximately 1 year in a modified FB-Line with the same capability. Some of the necessary facility modifications and dissolution operations could take place in parallel. However, even if DOE can find or develop a container suitable for transport of the plutonium solutions, the total time required to convert and package the plutonium contained in the Mark-31 targets into an oxide using this variation would be over 9 years (as opposed to 4 years using a modified FB-Line). For the above reasons, DOE did not consider these two variations to be reasonable oxide alternatives and warrant detailed analysis.
· Improving Storage.
DOE would move all Mark-31 targets to the L-Reactor Disassembly Basin and continue to store them there while it constructed a new Dry Storage Facility. The no-action activities described for stable material (see Section 2.1) would be applicable for these targets during the time DOE was constructing the new facility.
· Processing and Storage for Vitrification in Defense Waste Processing Facility.
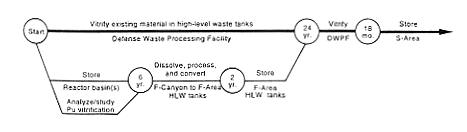
DOE would continue to store the Mark-31 targets until it was ready to transfer material to the high-level radioactive waste system. DOE would process the existing depleted uranium solutions in F-Canyon through the FA-Line to make room for processing the Mark-31 targets. The resulting depleted uranium oxide would be loaded in 208-liter (55-gallon) drums and placed in storage. In F-Canyon, DOE would dissolve the targets and then process the material to separate the plutonium from the depleted uranium. Then, rather than transferring the plutonium solution to FB-Line, DOE would poison, concentrate, and neutralize the solution and discharge the mixture to the F-Area high-level waste tanks. DOE would vitrify the material at the proposed Defense Waste Processing Facility; the difficulties associated with this process would be the same as those described in Section 2.3.1 for the H-Canyon plutonium solutions. The depleted uranium would be converted to an oxide in FA-Line, packaged, and placed in storage. The high-level waste generated during the chemical separation and chemical adjustment operations would be sent to the F-Area high-level waste tanks.
· Vitrification (F-Canyon).
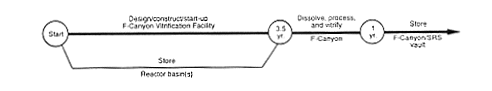
DOE could use the proposed F-Canyon Vitrification Facility to vitrify the plutonium in the Mark-31 targets. DOE would continue to store the material until the new facility was available. Then the material would be transferred to F-Canyon and dissolved. The material would be processed to separate the depleted uranium from the plutonium, and the plutonium would be vitrified. The depleted uranium solution would be converted to depleted uranium oxide in FA-Line. Any high-level waste from these operations would be transferred to the F-Area high-level waste tanks.
· Continuing Storage (No Action). DOE would continue to store the Mark-31 targets in the water-filled basins. The no-action activities described for stable material (see Section 2.1) would be applicable for these targets.
DOE's preferred alternative is Processing to Metal. DOE anticipates that it would complete the stabilization activity in about 2-1/2 years, as opposed to 4 years for the Oxide Alternative and 4-1/2 years for the Vitrification (F-Canyon) Alternative. In addition, the Processing to Metal Alternative would rely the most on previously operated systems, equipment, and facilities. The Vitrification via the Defense Waste Processing Facility Alternative was not selected because stabilization activity could not be initiated within the next 10 years (or more) due to the technical issues and the inventory of existing high-level waste that would have to be vitrified first. The Dry Storage Facility required for the Improving Storage Alternative would not be available within 10 years.
As a precursor to the Processing to Metal, Processing to Oxide, Processing and Storage for Vitrification in the Defense Waste Processing Facility, and the Vitrification (F-Canyon) Alternatives, DOE could dissolve unirradiated depleted uranium targets (which would result in no fissile material or fission products) in the F-Canyon dissolvers as part of equipment testing and operator training evaluations.
2.3.5 MARK-16 AND -22 FUELS
Approximately 1,900 irradiated fuel assemblies are stored in water-filled basins in the K-, L- and P-Reactor areas and in the H-Canyon facility. The fuel tubes contain highly enriched uranium and are clad in aluminum. DOE has identified the following alternatives for management of these fuels:
· Blending Down to Low-Enriched Uranium.
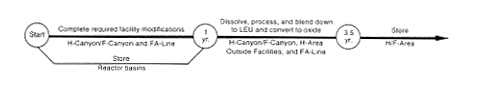
DOE would load the fuel tubes from the disassembly basins into casks, transport the casks to H-Canyon, dissolve the fuels, and separate enriched uranium from fission products, neptunium, and the small quantities of plutonium normally found in the fuel. This would be accomplished using the normal H-Canyon process. The fission products and other impurities would be transferred to the H-Area high-level waste tanks. The enriched uranium would be blended with depleted uranium and stabilized, as described in Section 2.3.2.
If DOE selected this alternative for the uranium solutions in H-Canyon and the Mark-16 and Mark-22 fuel, it would build only one storage facility, which would have an area of about 557 square meters (6,000 square feet).
As a variation to this alternative, DOE could transport the fuel to F-Canyon for processing. In this case, the blending operations would occur immediately after the fuel dissolving operations. Depleted uranium from FA-Line or from material already in the canyon would be added after the dissolution process. The resulting low-enriched uranium would be separated from the other material and radioactive decay products in the fuel and transferred to FA-Line for conversion to uranium oxide. The oxide would be stored in 208-liter (55-gallon) drums. The fission products and other materials would be transferred to the F-Area high-level waste tanks.
· Processing to Oxide (Uranium Solidification Facility).
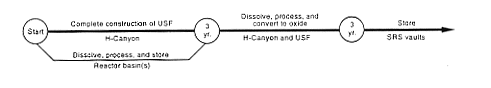
DOE would continue to store the fuel while completing construction of the Uranium Solidification Facility in H-Canyon. After construction, DOE would process the fuel as described in Section 2.3.2, transfer the resulting enriched uranium solution to the Uranium Solidification Facility, convert the uranium solution to an oxide, package the oxide, and place the containers in a vault for storage.
· Improving Storage.
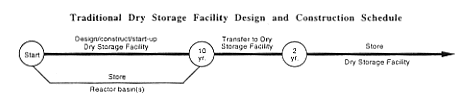
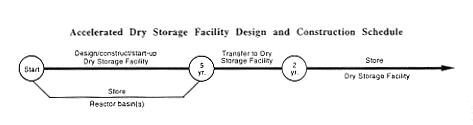
While constructing the new Dry Storage Facility, DOE would manage the Mark-16 and -22 fuel as described in Section 2.1 for no-action activities. Then DOE would transfer the fuel to the completed facility. DOE estimates that movement of the Mark-16 and -22 targets to the new facility would not begin for at least 10 years.
· Processing and Storage for Vitrification in Defense Waste Processing Facility.
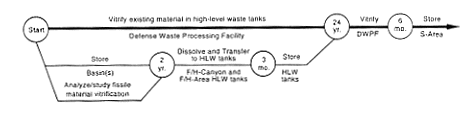
DOE would continue to store the material in solid form until it could complete technical studies on the transfer of fissile solutions to the high-level waste tanks. When the studies were complete, DOE would move the material to H-Canyon and dissolve it, adjust the resulting solution to ensure the nuclear criticality safety of the material in the waste tanks, and vitrify the material at the proposed Defense Waste Processing Facility. The difficulties associated with this process would be the same as those described in Section 2.3.1 for the H-Canyon plutonium solutions.
· Continuing Storage (No Action). DOE would continue to store the Mark-16 and -22 fuel in a water-filled basin. The no-action activities described for stable material (see Section 2.1) would be applicable for the fuel.
DOE's preferred alternative is Blending Down to Low-Enriched Uranium. DOE anticipates that it could complete this alternative about 2 years more quickly than Processing to Oxide (Uranium Solidification Facility), for which it would have to build the Uranium Solidification Facility. The Vitrification via the Defense Waste Processing Facility Alternative was not selected because stabilization activity could not be initiated within the next 10 years (or more) due to the technical issues and the inventory of existing high-level waste that would have to be vitrified first. In addition, DOE did not select the Improving Storage Alternative because it does not expect the Dry Storage Facility to be available within 10 years.
DOE did not evaluate Processing to Metal because this capability does not exist at the SRS and, because the oxide form of the material would be stable, there would be no advantage in developing the capability to produce uranium metal.
2.3.6 OTHER ALUMINUM-CLAD FUEL AND TARGETS
Approximately 900 metal fuel and target elements are stored in water-filled basins in the K-, L-, and P-Areas. These elements contain small amounts of fissile material; primarily they contain such materials as thorium, cobalt, and thulium. DOE has identified the following reasonable alternatives for management of these fuels and targets:
· Processing and Storage for Vitrification in Defense Waste Processing Facility.
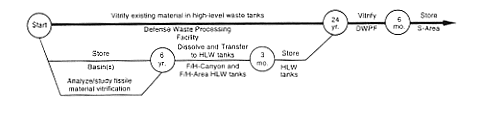
DOE would continue to store the material in its current form until it could complete technical studies on the transfer of fissile solutions to the high-level waste tanks. DOE anticipates that these studies would be simpler than those for other material evaluated in this EIS because the fissile material content of these items is relatively low. When the studies were complete, DOE would move the material to a B-Line or canyon and dissolve it. DOE would adjust the resulting solution to ensure the safety of the material in the waste tanks from nuclear criticality. The material would be vitrified at the proposed Defense Waste Processing Facility.
Improving Storage.
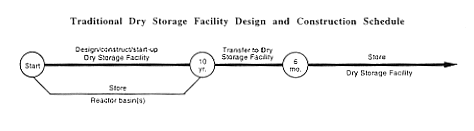
While constructing the new Dry Storage Facility, DOE would manage the fuel and targets as described in Section 2.1 for no-action activities of Section 2.1. Then DOE would transfer the material to the new Dry Storage Facility, which would not be available for about 10 years.
· Continuing Storage (No Action). DOE would continue to store the fuel in a water-filled basin. The no-action activities described for stable material (see Section 2.1) would be applicable for this fuel.
DOE proposes Processing and Storage for Vitrification in the Defense Waste Processing Facility as the preferred alternative because the relatively small amount of fissile material in the fuel would reduce the criticality concerns associated with using this method. DOE did not evaluate in detail alternatives that involved chemical dissolution and separation because the amount of fissile material would be so low there would be very little to recover, and therefore, the net result would be the same as the Processing and Storage with Vitrification in the Defense Waste Processing Facility Alternatives (i.e., the material would be dissolved and discharged to the high-level waste tanks).
2.4 Comparison of Alternatives
DOE would select a management alternative for each category of nuclear material listed in Table 2-1. This would result in the implementation of a specific combination of the alternatives described and analyzed in this EIS. Tables 2-2 through 2-11 compare the environmental impacts for each alternative by nuclear material type and summarize how each alternative compares to the others. Choosing No Action for the management of each nuclear material group is likely to result in the smallest impacts for the 10-year period. Taking action to stabilize materials would entail some increased exposure and risk compared to No Action during the 10-year period. However, over the long term, choosing No Action could result in greater impacts than those that would occur by
choosing another alternative. This is because choosing No Action would result in the need for greater management vigilance and consequent worker exposures and because of the increased possibility that continued changes in material chemistry could result in releases to the environment. Furthermore, DOE eventually would have to take some type of stabilization action, and the attendant risks and exposures from these actions would occur at that time.
2.5 Other Factors
The selection of scenarios for the stabilization of SRS nuclear materials depends in part on existing technology (or on technology that DOE could develop quickly), the capabilities of existing SRS facilities, and the extent to which the actions would support long-term storage objectives. Consistent with a comprehensive review of options for plutonium disposition, DOE will consider the technical, nonproliferation, environmental, budgetary, and economic aspects of each alternative in each scenario before it selects any alternative for implementation.
In addition to comparing scenarios against the environmental criteria listed in Section 2.4, DOE has compared other factors related to the stabilization of nuclear materials. These factors are representative of issues addressed by the National Academy of Science in its study of the management and disposition of plutonium (NAS 1994), the Office of Technology Assessment plutonium study (OTA 1993), and comments received during the EIS scoping period.
2.5.1 NEW FACILITIES REQUIRED
This factor considers qualitative impacts on the number and size of new facilities required, and the probable long-term restoration requirements after their use. All alternatives for candidate plutonium materials for stabilization, except Continuing Storage (No Action), would involve constructing the proposed Actinide Packaging Facility or modifying an existing facility inside the F-Area fence. Therefore, only this construction differentiates between Continuing Storage and the other alternatives. Continuing Storage would be the most advantageous alternative for this factor.
Processing H-Canyon uranium solutions and Mark-16 and -22 fuels to oxide in FA-Line would involve the construction of a new small storage building for low enriched uranium inside the F-Area fence. Processing these materials to oxide in H-Area would involve completing construction of the Uranium Solidification Facility. Therefore, processing these materials to oxide would be less advantageous than other alternatives for this factor. In addition, because the F-Area construction would be less costly and time-consuming than completion of the Uranium Solidification Facility, this factor would differentiate between these alternatives.
Vitrification in F-Canyon would involve preparing the F-Canyon Vitrification Facility to add vitrification and bagless transfer capability. Processing plutonium to an oxide in FB-Line would involve modifying FB-Line from its current metal-producing configuration.
Finally, Improving Storage of Mark-31 targets, Mark-16 and -22 fuels, and other aluminum-clad fuel and targets would involve constructing a new Dry Storage Facility on an undeveloped site. This construction makes this the least advantageous alternative for these materials.
2.5.2 SECURITY AND NONPROLIFERATION
This factor relates to how well each alternative would support national security objectives and nonproliferation. This issue is being debated on the national and international levels, and consensus has yet to be reached. However, DOE has qualitatively evaluated the alternatives and compared them to one another.
All the alternatives would involve the use of facilities within controlled industrial areas of the SRS, which are supported and protected by an armed protective force. However, the solutions or stabilized forms of plutonium would have varying degrees of utility in potentially supporting or leading to the manufacture of a nuclear weapon.
The Processing and Storage for Vitrification in the Defense Waste Processing Facility Alternative would produce a material form that would be least attractive for use in producing a nuclear weapon. Therefore, it would represent the most advantageous alternative in this regard. The Processing to (plutonium) Metal Alternative would result in a chemical form that closely resembled that used in weapons production. The other alternatives evaluated would maintain or convert plutonium to forms that would require varying degrees of processing to produce a form suitable for weapons use. All the alternatives would involve the use of facilities inside controlled industrial areas of the SRS, which are supported and protected by an armed guard force.
DOE has committed to prohibit the use of plutonium-239 or weapons-usable highly enriched uranium separated or stabilized during the phaseout, shutdown, and cleanout of weapons complex facilities for nuclear explosive purposes (DOE 1994c).
2.5.3 IMPLEMENTATION SCHEDULE
Of the stabilization alternatives, those chosen for the Preferred Alternatives Scenario could be implemented in the shortest period of time. Alternatives involving dry storage would add the longest lead time (10 years), and the Processing and Storage for Vitrification in the Defense Waste Processing Facility Alternative would add at least 9 years of preparation.
2.5.4 TECHNOLOGY AVAILABILITY AND TECHNICAL FEASIBILITY
This factor relates to the extent that technology development would be required and its likelihood of success. Processing to Metal in F-Area and Processing to Oxide in H-Area represent the most technically proven of the stabilization alternatives; they would use existing technology and equipment. The Vitrification (F-Canyon) and Processing and Storage for Vitrification in the Defense Waste Processing Facility Alternatives appear to be technically feasible, but would require increasing amounts of technology development. Dry storage would involve the most technology development.
In general, the technical uncertainty would increase as the stabilized form differed from that historically produced. There would also be technical uncertainty about the continued storage of the plutonium solutions under the Continuing Storage Alternative as a result of radiation and chemically induced changes in the solution chemistry and form.
2.5.5 LABOR AVAILABILITY AND CORE COMPETENCY
There would be differences between the level of personnel knowledge and training required for each alternative. In addition, there would be impacts from providing the needed level of training. In general, the Processing and Storage for Vitrification in the Defense Waste Processing Facility Alternative would require the most labor to implement (due to the combination of a long period of maintaining stored materials plus processing activity). The Continuing Storage and Processing to (plutonium) Metal Alternatives would involve activities similar to those performed in the past; as a result, facility personnel would have existing training and qualification programs to maintain core competency. The Processing to (plutonium) Oxide, Vitrification, and Improving Storage Alternatives would require additional levels of training; the only impact anticipated from such additional training would be the incremental funding and time required.
2.5.6 AGING FACILITIES
All the alternatives would involve the use of existing facilities, some of which have been in operation for more than 40 years (e.g., F-Canyon). The No-Action Alternative would require continued storage of the material in existing facilities and is, therefore, the least desirable or advantageous in this regard.
Although the Processing and Storage for Vitrification in the Defense Waste Processing Facility Alternative would eventually make use of the proposed DWPF, it would require maintenance of the solutions in F-Canyon for 6 to 9 years. In addition, it would involve the transfer of the plutonium solutions to the high-level waste tanks. Therefore, this alternative has only a slight advantage over the No-Action Alternative.
While the Processing to (plutonium) Metal Alternative would involve limited use of the F-Canyon and FB-Line for stabilization, it would involve continued storage of the metal in the FB-Line vault. Therefore, it represents some reliance on aging facilities, but also represents an advantage over the No-Action and Vitrification Alternatives.
The Processing to (plutonium) Oxide Alternative would involve limited use of the F-Canyon and FB-Line facilities. It could use a new or modified facility for conversion to a high-fired oxide and eventual storage. The use of a new facility would represent the minimum reliance on existing or aging facilities.
2.5.7 MINIMUM CUSTODIAL CARE
The vitrification alternatives would eventually result in a stabilized form of material that would require a minimum of custodial care. However, continued custodial care of the materials would be required in canyons, vaults, or high-level waste tanks until vitrification had been accomplished. Continued Storage would involve maintaining candidate materials for stabilization (necessitating increasing surveillance, maintenance, and corrective actions) for the longest time and, therefore, can be considered the least advantageous alternative in this regard.
Other processing and improving storage alternatives would have varying levels of custodial care requirements. Stable materials would need less care than candidate materials for stabilization, so the preferred alternatives would involve less custodial care than other alternatives because they would stabilize the materials the earliest.
2.6 Other Activities for Reduction of Risk
DOE identified several alternatives that it eliminated from detailed study because they increased environmental or other risks without commensurate benefits or because they would be inconsistent with National Environmental Policy Act requirements for interim actions. These include processing to include fission products, transporting material off the Site, and burial.
DOE considered the addition of fission products to increase the radioactivity of the stabilized form of the material (e.g., metal). Such an addition would make the material essentially "self-protecting" from theft or potential use in weapons because of high radiation levels. However, this method would result in increased exposures to personnel performing processing and handling operations (e.g., at FB-Line). DOE considers such increased exposures to personnel to be unwarranted and, therefore, did not consider this a reasonable alternative.
Offsite transportation and onsite burial could reduce SRS risks but are disposition alternatives that could limit the choices of alternatives in the ongoing "Programmatic Environmental Impact Statement for Storage and Disposition of Weapons-Usable Fissile Materials" (59 FR 31985). This would be contrary to National Environmental Policy Act requirements and, therefore, DOE did not consider this a reasonable alternative.
REFERENCES
DOE (U.S. Department of Energy), 1994a, DOE Standard: U.S. Department of Energy Criteria for Safe Storage of Plutonium Metals and Oxides, DOE-STD-3013-94, Washington, D.C.
DOE (U.S. Department of Energy), 1994b, Assessment of Interim Storage of Plutonium Solutions in F-Canyon and Mark-31 Targets in L-Basin at the Savannah River Site, DOE EH-0397P/
SRS-FCAN-94-01, Office of Environment, Safety and Health, Washington, D.C.
DOE (U.S. Department of Energy), 1994c, "ACTION: Commitment To Prohibit the Use of Plutonium-239 and Highly Enriched Uranium Separated and/or Stabilized During Facility Phaseout, Shutdown and Cleanout Activities for Nuclear Explosive Purposes," memorandum to the Secretary of Energy from Assistant Secretary for Defense Programs and Assistant Secretary for Environmental Management, Washington, D.C., December 20.
NAS (National Academy of Sciences), 1994, Management and Disposition of Excess Weapons Plutonium, Committee on International Security and Arms Control, National Academy Press, Washington, D.C.
OTA (Office of Technology Assessment), 1993, Dismantling the Bomb and Managing the Nuclear Materials, Office of Technology Assessment, U.S. Congress, Washington, D.C.
|
NEWSLETTER
|
| Join the GlobalSecurity.org mailing list |
|
|
|






