NATO HANDBOOK ON THE MEDICAL ASPECTS
OF NBC DEFENSIVE OPERATIONS
AMedP-6(B)
PART III - CHEMICAL
ANNEX A
BIOCHEMICAL ACTION OF NERVE AGENTS
TABLE OF CONTENTS
Paragraph A.01. Acetylcholinesterase
Figure A-I. Hydrolysis of Acetylcholine
A-II. Overall Reaction Occurring During Hydrolysis of Acetylcholine>
ANNEX A
BIOCHEMICAL ACTION OF NERVE AGENTS
A.01. Acetylcholinesterase.a. Acetylcholinesterase exists as a large, complex molecule. The active centre has been suggested to comprise:
(1) A negatively charged or anionic sub-site.
(2) An esteratic sub-site.
b. The current model for the binding group at the esteratic site is an hydroxyl group of a serine molecule of the enzyme. This group binds to the acyl carbon atom of acetylcholine. The sequence of reactions taking place during the hydrolysis of ACh is shown in Figure A-I.
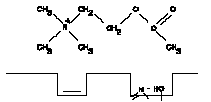
1. Acetylcholine molecule and active site of enzyme shown together but not having undergone any interaction.
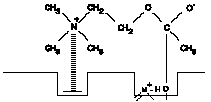
2. Acetylcholine combined with enzyme to form a substrate-enzyme intermediate (short lived).
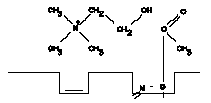
3. The ester link in the acetylcholine has been broken and free choline has been formed.
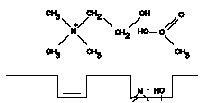
4. The acyl group has become detached from the enzyme leaving: choline, acetic acid, and the enzyme returned to its original state.
c. The overall reaction could be represented by Figure A-II.
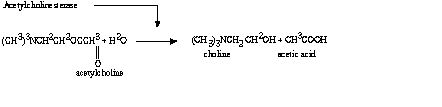
Figure A-II. Overall Reaction Occurring During Hydrolysis of Acetylcholine
d. The choline is taken up by the pre-synaptic or pre-junctional nerve terminals and recycled by combination with acetyl CoA catalysed by the enzyme choline acetyltransferase to form more acetylcholine.
e. The destruction of acetylcholine by Ache is a very rapid reaction. Organophosphorus compounds (including the nerve agents) act by combining essentially irreversibly with acetylcholinesterase molecules and preventing their activity. The combination of GB with AChE is shown in the series of reactions in Figure A-III.
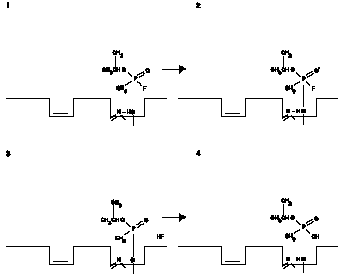
Figure A-III. GB and AChE Reactions
f. Unlike the hydrolysis of acetylcholine, however, this series of reactions is slow as a result of the very slow, and rate limiting, final step (3-4) in Figure A-II. This stage takes place so slowly that in practical terms no regeneration of enzyme takes place and if the dose of organophosphate is sufficiently large the patient dies.
g. Another reaction may be added to the above series: the reaction describing the process of AGING 11:
 R.(OH)PO enzyme + R'OH.
R.(OH)PO enzyme + R'OH.h. This loss of an alkyl group produces a very stable agent-enzyme complex which is then resistant to spontaneous hydrolysis and reactivation by oximes. The rate of aging is dependent on the nature of the alkyl group and is fairly slow (hours) in the case of GB or VX but is very rapid (minutes) in the case of GD.
i. Aging is the main cause of failure of compounds designed to speed reactivation of the enzyme to be of as much value in GD poisoning as they are in the treatment of GB or VX poisoning.
|
NEWSLETTER
|
| Join the GlobalSecurity.org mailing list |
|
|
|

