CHAPTER 1
Water Treatment
Section I
QUALITY
CHARACTERISTICS
Water quality has two major areas of concern as it relates to the daily operation of a water point. The first is the quality of the raw water source and how it affects the operation of the water purification equipment and use of treatment chemicals. The second is the quality of the product water and surveillance techniques used to guarantee its potability.
The nature of the raw water source will dictate the amount of water each purifier can produce. The total daily water requirement will indicate if additional water purification and storage equipment is needed to meet the demand. TB MED 577 lists the maximum concentration allowed for various chemicals in raw water sources. Concentrations above these limits eliminate a source as a potential water point for military operations. FM 10-52 describes in detail the effects and possible origins of physical and chemical raw water contaminates.
Chemical analyses and microbiological examinations of raw and treated water are required on a routine basis at water point sites. Chemical tests are necessary to ensure correct operation of the water purification equipment. Conduct chemical analyses during treatment to ensure proper chemical dosages and that the product water is potable. Conduct microbiological examinations after treatment to determine potability of the water.
WATER QUALITY ANALYSIS UNIT
The WQAU gives the water treatment operator the ability to rapidly detect five water quality parameters: temperature, pH, total dissolved solids, turbidity, and free available chlorine (chlorine residual).
The WQAU consists of an electronic analytical device, an internal power source, basic spare parts, and the M272 Water Testing Kit-Chemical Agent. The WQAU weighs less than 40 pounds, has a volume of less then 2 cubic feet, and requires less than five minutes to measure the five separate parameters.
The WQAU can operate in geographical areas where air temperatures range from -28°F to 120°F. It is used primarily by water purification personnel that operate DS and GS water purification equipment. Water purification personnel use the unit during water point reconnaissance missions to assess the suitability of raw water sources and during water purification operations to assess the quality of finished water.
APPLICATION
The following sections are summaries of the effects the five physical and chemical characteristics of water have on the four treatment processes Army water purification units use to treat water (coagulation and flocculation, filtration, reverse osmosis, and disinfection). The water treatment specialist must know how to monitor for and respond to the presence or absence of these characteristics if he is to properly operate water purification, storage, and distribution equipment.
Section II
COAGULATION AND FLOCCULATION
RAW WATER IMPURITIES
Impurities present in raw water are in suspended, colloidal, and dissolved forms. These impurities are dissolved organic and inorganic substances, microscopic organisms, and various suspended inorganic materials. You must destabilize and bring together (coagulate) the suspended and colloidal material to form particles. Remove these particles by filtration. Colloidal material is the most difficult to remove from raw water. Processes that remove colloids from water also remove suspensions.
PROPERTIES OF COLLOIDS
Size and electrical charge are properties of colloids. They are explained below.
Colloids have an extremely small size (approximately 0.0001 to 1.0 microns) and a large surface area in relationship to their weight. Because of these factors, they do not readily settle out of solution.
Electrical Charge
Most colloids found in water show a negative charge. Because like charged particles repel, colloidal material will not join to form suspended particles unless the particles' electrical charge is reduced or neutralized.
COAGULATION PRINCIPLES
In coagulation, a chemical (polymer) is fed to the raw water to neutralize and destabilize particle charges on the colloids. Destabilized colloidal particles adhere to each other. Because many colloidal particles are present in the water, charge neutralization among all particles requires immediate and even dispersion of the coagulant. The destabilization reactions occur very rapidly. Therefore, incomplete or slow mixing results in wasted chemical and uneven flocculation.
FLOCCULATION PRINCIPLES
Once colloidal destabilization has occurred, random particle motion causes particle collision, resulting in formation of a larger particle or floc. These neutralized particles stick together forming floc masses. Bacteria particles are also neutralized (but not physically deactivated) and become entangled in the floc. As this massing continues, particle size and weight increase to a point where the larger floc can be removed by filtration (Figure 1-1 and Figure 1-2).
FACTORS INFLUENCING COAGULATION
Turbidity, pH, and color influence the coagulation of raw waters. They influence the type and amount of chemicals required. It is necessary to know which chemicals produce the most satisfactory results and to determine the exact dosage required. This can be done by test analysis or by trial runs of the equipment. The following information is essential for controlling turbidity, pH, and color in the water purification process.
The type of turbidity plays a role in determining needs for the best pH and coagulant dosage. The following generalizations can be made:
- Turbidity caused by clay concentrations requires a minimum of coagulant to provide an entangled mass of floc.
- As turbidity increases, an additional coagulant dosage is generally required, although the dosage of coagulant does not increase in direct proportion to the increase in turbidity. Highly turbid waters require relatively lower coagulant dosages than low turbidity water due to the higher probability of particle collisions.
- The organic matter typically adsorbed on clays in natural stream water does not significantly increase coagulant demand.
- Organic colloids caused by sewage and industrial wastes are more difficult to coagulate because of extensive chemical reactions that occur between the coagulant and the colloidal organic matter.
Test for Turbidity
Determining turbidity is perhaps the most important control test performed. Perform turbidity tests on raw and treated water to determine overall turbidity removal effectiveness.
Once you determine chemical dosages, perform raw water turbidity tests as often as the raw water quality varies. River water requires more frequent analysis than lake water, especially during the spring when thawing snow and runoff dramatically increase the silt load in rapidly moving rivers. Well water is not usually turbid.
Conduct turbidity analysis on treated water. An increase in effluent turbidity indicates process control problems and may be related to filter breakdown, poor floc formation, or coagulant more than any other test, indicates the effectiveness of the colloid removal process.
pH Range
Coagulant chemicals have an optimum pH range in which good coagulation and flocculation occur in the shortest time with a given dosage. The type of colloid in the water also affects the pH range for efficient coagulation. In some cases, it may be necessary to use chemicals to adjust the pH of the water to obtain the best coagulation and flocculation. Trial and error testing is the only sure method to determine the most efficient coagulant and pH range for the particular water dosage variation. The filter effluent turbidity test, being treated.
Color
Determine raw water color visually to note any dramatic color changes. Take the proper corrective action (for example, pH adjustment, coagulant dosage change). Color usually increases during spring runoff and again in the fall from surface sources. Color removal deserves special consideration due to special colloidal properties.
Cause. Organic color is primarily caused by the decomposition of natural organic matter. Oxidized, metallic ions (such as iron) produce nonorganic color.
Size. Color-associated colloidal particles are the smallest of the turbidity-causing particles. They generally have diameters of about 0.003 microns, as compared to clays with a typical diameter of 1.0 micron.
Charge. Color particles are negatively charged, with the strength of charge depending on pH. The charge is an integral part of the molecule, rather than merely adsorbed on the surface as with clays.
Removal. The mechanism of color removal is the direct formation of an insoluble chemical compound with a metallic coagulant (such as ferric chloride), rather than charge neutralization usually associated with turbidity removal by polymers. Color removal is affected by pH and should be carried out under acid conditions (pH 4 to 6). Raising the pH for other processes before removing color may cause color fixation to take place. This makes subsequent color removal (even at optimum pH) more difficult.
Physical Factors
Physical factors include temperature, nuclei present, and flash mixing. These factors are explained below.
Temperature. As the temperature decreases, the water viscosity increases. The rate of floc settlement decreases as the water viscosity increases, resulting in general loss of coagulation and flocculation efficiency. Temperature effect can be overcome by adding more chemical coagulant or by operating the coagulation process at the best pH for the particular coagulant being used.
Nuclei present. Although coagulation and floc formation can occur in the complete absence of solid suspended particles, the presence of suspended particles serves to increase the rate of flocculation, density of floc, and removal efficiency.
Flash mixing. Flash mixing is the very rapid and highly turbulent agitation that uniformly disperses the coagulant and promotes coagulant contact with turbidity particles. Flash mixing is usually maintained for 30 to 60 seconds before filtration.
CHEMICAL AIDS
There are a number of chemicals that can be used as aids to coagulation and flocculation. Their characteristics and effects and some precautions for their use are discussed below.
Polyelectrolytes are manufactured synthetically as a high molecular weight, colloidal-size molecule. The molecule can be manufactured to meet varying specific needs. Synthetic polyelectrolytes are available in anionic (negatively charged), cationic (positively charged), or nonionic (neutral) forms. The anionic and nonionic forms are generally used as flocculent aids, while cationic forms are generally used as primary coagulant or coagulant aids. The polymer used by Army ROWPUs is of the cationic form. In concentrated liquid form, polymers are reasonably stable and can be stored one year. In feed solution form, however, shelf life is only about 24 hours. Polymer solution strengths are usually 1 percent or less since more concentrated solutions can start to solidify, causing clogging of feeding devices and valves.
Polymers produce a very large and dense floc that can greatly increase the particle removal rate. The floc produced is often stronger and more easily removed by filtration. Polymers are less easily influenced by water pH, alkalinity, hardness, and turbidity than are other chemical aids. Polymers are easy to prepare, use, and store.
Overdosage of polymers can hinder flocculation. Determine precise dosages of polymers by trial and error testing procedures. Only those polymers specifically approved by the USEPA are acceptable for use in potable water treatment used in Army field water operations.
Section III
FILTRATION
FILTRATION PROCESSES
Filtration processes remove microorganisms and other suspended matter from the product water. The suspended material consists of floc from chemical clarification. The removal of these suspended solids is essential, as the feedwater for the reverse osmosis must be as free from suspended solids as possible. The following is an overview of the principles of filtration.
It is a common misconception that filtration removes suspended solids by a simple straining process where particles too large to pass through openings in the filter media are retained on the media. Actually, the mechanisms involved in removing suspended solids by filtration are very complex. While straining is important at the filter media surface, most solids removal in deep granular filters occurs within the filter bed.
Flocculation and sedimentation in the pore spaces between filter media particles is an important removal mechanism as well as adsorption of particles onto the filter media surface. Additional straining between media particles within the filter also contributes to overall solids removal.
Proper treatment of the influent water to the filters is essential for good filter performance. If not coagulated, fine turbidity will pass through a filter. Also, if large coagulated flocs are allowed to develop, they will tend to forma thick layer on top of the filter media, causing substantial resistance to flow and rapid head loss.
Head Loss Development
As floc particles accumulate at the influent surface and in the internal pores of the filter bed, resistance to flow is created and loss of hydraulic head through the bed increases. Backwash the filter when the head loss exceeds a predetermined value. Clean it if the high pressure applied to force water through the bed shears the accumulated flocs, causing them to pass through the filter and create a turbid effluent.
Solids Breakthrough
Filter operation and performance are adversely affected by hydraulic surges. The higher rate of flow through the filter bed causes increased velocities between media particles, shearing the accumulated floc particles and carrying them deeper into the filter bed or all the way through the filter.
CLASSIFICATION OF FILTERS
Classify filters by the media type, the flow rate, the direction of flow, the hydraulics (gravity or pressure), and the method of controlling the flow through the filter. The most common method of classifying filters, however, is by the type of filter medium. The two types used in Army water purification equipment are the multimedia and the cartridge.
Multimedia Filters
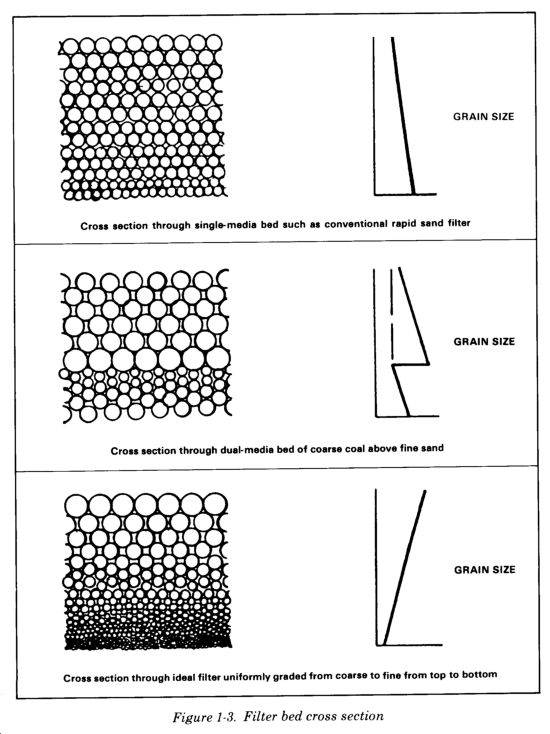
These filters use sand and crushed anthracite coal on a graded gravel base. Media layers are arranged in a course to fine gradation in the direction of flow, which allows greater depth of penetration of floc particles. Multimedia filters are selected with specific gravities so that moderate intermixing between media layers occurs during backwashing. If the different media were in sharply stratified layers, all solids removal would occur due to straining at the tope surface of each layer. With intermixing, the upper zones of the fine media mix with the lower ones of the coarse media, resulting in a filter bed with more overall filtering capacity (Figure 1-3). Filter materials are rated by particle size and uniformity. Typical characteristics are listed in Table 1-1. Filter media characteristics are explained below.
Sand. The types of sand used in filtration are silica and garnet. Garnet sand is denser and smaller than silica sand and is used in the final (bottom) layer in multimedia filters.
Anthracite coal. Crushed anthracite coal is a more angular media with higher porosity than sand, with a resulting higher storage capacity for flocculated solids. The lower specific gravity (density) of the anthracite compared with sand allows the anthracite to maintain its position in the filter bed following backwashing.
Filter gravel. Use silica gravel for a thick base layer to prevent the filter media from being washed out of the filter and to distribute the flow of backwash water. The gravel acts as a buffer zone, protecting the filter media from localized high-velocity streams during backwash. Gravel sizes range from a maximum of 1 to 2 inches to a minimum of about 1/16 inch. To prevent the gravel from being mixed with the overlying media by high-velocity streams, place a layer of dense, coarse garnet sand between the gravel and the fine media.
Cartridge Filters
Select cartridge filters on the basis of a desired filtration performance need. Understanding the important filtration performance variables, such as particle size removal efficiency, filter service life, permeability and system compatibility, is the best way to obtain desired performance.
Filter types. There are two types of cartridge filtration: depth filtration, in which solid particles become trapped within the filter medium, and surface filtration, in which solid particles form a cake on the surface of the filter medium. Wound fiber cartridges function primarily as depth filters and are the standard cartridge used in Army purification units. Pleated cartridges primarily act as surface filters. In every filtration application, both surface filtration and depth filtration will take place simultaneously. However, generally one is more important than the other. Wound and pleated cartridges also perform differently in other ways. Differences in filter cartridge permeability, available materials of construction, ability to withstand pulsating flow conditions, and particle removal efficiency are just a few examples where wound and pleated cartridges may not perform quite the same. The following will examine the advantages and limitations of wound cartridge filters, as this is the type used in military water purification equipment. This manual reviews cartridge design, capture mechanisms, micron ratings, and performance.
Fibrous filter design. Wound and pleated cartridge filters separate suspended particles from a fluid by passing the fluid through a porous fibrous medium. Therefore, these cartridges perform according to the laws that govern fibrous filter structures. There are three main variables that affect the design of fibrous filters. They are fiber diameter, porosity or void volume, and thickness. A change in any one of these three variables will also affect the different performance characteristics of wound and pleated filters. These variables are also responsible for the subtle differences that produce different particle removal efficiencies within each type of cartridge.
Wound cartridge design. A wound fiber cartridge has 3/4 inch of yarn continuously wound around a perforated center support core. The yarn may be cotton, polypropylene, rayon, acrylic, polyester, nylon, fiberglass, or Teflon. The yarn consists of intertwined fibers that are 15 to 20 microns in diameter. These fine fibers are important in obtaining small, pore-size openings. Wound fiber cartridges are available in various lengths and micron ratings. Other important design features include the availability of various center core materials for fluid compatibility, core covers and end treatments to reduce the possibility of media migration, and extended cores to improve cartridge sealing (Figure 1-4).
Capture mechanisms. Remove suspended solid particles from a fluid stream by a filter in one of two ways. First, the particles may simply be too large to pass through the filter flow channel opening or pore. The particle is physically restrained by the filter medium or its extension, the filter cake. This kind of direct particle interception by the filter is an example of mechanical capture. A screen or sieve traps particles on its surface in this way. Mechanical capture is also a characteristic of pleated cartridge filters. Mechanical capture by sieving generally occurs at the filter's surface. However, particles can also penetrate the larger surface pores only to be sieved by smaller pores within the successive inner layers of the filter. This internal sieving is likely to occur when the filter medium has a broad pore-size distribution, not unlike that found in wound fiber cartridges. The second type of filter capture mechanism occurs when a particle enters a filter opening, or pore, larger than itself. Under the influence of inertia, diffusion, or other forces, the particle collides with the pore wall or interior pore structure. Once the particle contacts the interior pore surface, it sticks to that surface. This type of capture mechanism is known as adsorptive capture. There are several attractive forces that may be responsible for retaining a particle on the interior pore surface. These include electrostatic (opposite charge), hydrogen bonding, and hydrodynamic forces.
Wound cartridge workings. Mechanical and adsorption capture mechanisms are present in every filtration application, whether wound or pleated cartridges are used. However, a wound fiber cartridge, with its substantial media thickness and graded density, accentuates adsorptive capture mechanisms. The wound fiber cartridge design creates a tortuous, fluid flow passage. Suspended particles entering the filter's matrix are subjected to endless fiber impact and internal sieving obstructions. This circuitous pathway is effective in retaining deformable particles. In these applications, it is important that the flow rate per length of cartridge be low to increase contact time with the filter medium. Particle capture occurs throughout the depth of the wound cartridge from the surface to the center core. When the particle-size distribution of the contaminant is broad, wound fiber cartridges have an excellent solids-holding capacity. This is due to the filter's high porosity (high internal void volume) and graded density design. Larger particles stop at or near the surface. Smaller particles are trapped at each of the successively denser layers. The result is substantial solids-holding capacity and a long service life. System operating conditions greatly influence wound fiber filter performance. Unsteady flow conditions may dislodge particles captured by adsorptive means. Too high a differential pressure across the filter media can drive a soft, deformable particle through the filter matrix. It is also important that wound filters be properly sized and operated under recommended flow rate conditions for longer service life. In most cases, the flow rate per 10-inch length of cartridge should not exceed 5 GPM. In fact, reducing the flow rate by a factor of five (for example, from 5 GPM to 1 GPM) will double the amount of solids a wound fiber cartridge can hold before reaching the recommended change-out differential pressure.
Micron ratings. All cartridge filters will remove a certain percentage of particles of each size that they receive in the fluid stream. A filter's micron rating is an indication of its removal efficiency performance. For it to have meaning, the micron rating of the cartridge must include the level of removal efficiency, usually expressed as a percentage, at the rated micron size. Wound cartridge filters used by the Army have 90 percent removal efficiency as their stated micron rating. While micron ratings corresponding to higher removal efficiencies are available, their filtration ability does not increase.
Cartridge filter performance. Permeability is a measure of the flow rate of a fluid through a porous medium at a specified pressure differential. It is the ease at which a porous medium will allow the passage of fluid. The filter medium thickness, surface area, porosity, and materials of construction affect the filter's permeability. Combined, these variables also dictate the pressure drop across the filter cartridge at a given flow rate and fluid viscosity. For example, the permeability of a filter cartridge would be stated as 3 GPM/10-inch length at 1 psid for a fluid with a viscosity of 1 centipoise. Permeability is inversely related to the removal efficiency of the filter medium. Cartridge filters with higher removal efficiencies are less permeable than those with lower removal efficiencies. In other words, a 1-micron cartridge has a greater resistance to flow when compared to a 50-micron cartridge. Operating wound fiber cartridges under high differential pressure or high flow rate conditions may adversely affect the life and removal efficiency of the filter.
Filter service life. Perhaps the most important filter performance relationship is the effect of flow rate on filter service life. As the flow rate per cartridge increases, filter service life decreases. The consequences of this relationship are economic and functional. Filter life is affected by initial differential pressure. To increase filter life, it is important to keep the initial differential pressure as low as possible. This is done by keeping the flow rate through the filter cartridge as low as possible. One method of reducing the flow rate per unit of filter area is to increase the number of cartridges while keeping the system flow rate constant. Increasing the number of cartridges from one to three triples the amount of surface area. Increasing the surface area by three may increase the life up to nine times. This is why all Army water purification units have multiple wound fiber cartridges. As discussed earlier, actual performance and service life can vary with system conditions and the nature of the solid particulate. However, it is important to remember that you can improve both filter performance and service life by lowering the flow rate per cartridge.
Flow Direction
You operate most granular filters with the direction of flow from top to bottom through the bed. This is because most filters, particularly older sand filters, use gravity for the hydraulic driving force. Some pressure sand filters operate in an upflow mode; however, these are exceptions.
Flow Control
The final classification of filters is based on the way you control the flow rate through the filter bed. There are three basic operating modes: constant rate, constant pressure, and variable declining rate.
Constant rate. Constant rate can be attained by using a flow control valve on the filter effluent. At the start of a filtration run, the media is clean and flow is too fast. for good filtration; therefore, the control valve is partially closed. As filtration continues and the media clogs with solids, the control valve gradually opens to maintain constant flow. An alternate method of constant rate filtration control in gravity filters is to build the filter cells with extra high walls and to split the flow equally between all filters. As the filter becomes clogged (causing resistance to flow), the water level above the media rises. This increases the head applied to the filter, thereby maintaining a constant flow rate.
Constant pressure. In constant pressure systems, the same hydraulic head is applied to the filter throughout the filtration run. Initially, the media is clean, resistance to flow is minimal, and the resulting flow rate is high. As the media clogs, the flow rate through the filter decreases. Pressure filters are similar to gravity filters in most aspects of construction and operation. The major difference is that the driving force applied to drive the water through the filter is the static pressure supplied by a pump. Filtration rates, methods of backwashing, and media are the same for gravity and pressure systems. Advantages of pressure filtration versus gravity are the higher head loss at which filters can be operated before backwashing is required, the higher residual head in the filter effluent line, and the reduced vertical dimensions of the filter. A major disadvantage is that the operator cannot watch the filter operations to monitor problems (for example, improper backwashing and mud ball formation).
Variable declining rate. Design of variable declining rate filtration systems has all filters fed from an oversized, common header. When one filter at a time is taken out of operation for cleaning or as a filter becomes fouled, the flow backs up through the common header and is applied evenly to the remaining filters, resulting in a slight, gradual increase in flow rate through all filters in operation. The filters are provided with extra depth above the media surface to allow the water level to rise as the media becomes clogged or when more water is applied as a result of backwashing a filter. The main differences between variable declining rate filtration and constant rate filtration with equal flow splitting are the location and type of influent arrangement and the head available for filtering.
FILTER OPERATIONS
Influent water to a filter discharges into a baffle to dissipate the velocity head that could disturb the surface of the filter media. The flow then passes through the filter media, gravel base, and drains. A rate controller is located in the effluent line from the filter to maintain a constant flow rate through the filter. Backwash the bed when the head loss through the filter bed increases above a certain level as a result of clogging of the media with removed solids, when effluent water quality fails to meet criteria, or when breakthrough of solids occurs. Backwashing is done by reversing the flow through the bed; this expands the media and shears loose the accumulated solids, which are carried away in the backwash water. Operating problems may include mud accumulation and permanent clogging of filter media, coating of media grains with various materials, improper coagulation, and backwash control. The single most common problem in filtration is unsatisfactory filter media cleaning.
Filter Rate
Maintain a relatively constant, or slightly declining, filter rate to prevent solids breakthrough. Sudden flow surges are not desirable as they may flush solids through the bed. Control the flow at the influent or effluent end of the filter.
Loss of Head
The resistance to water passing through the filter bed causes a loss of hydraulic head between the inlet and outlet of the filter. At the beginning of a filter run, suspended material collects primarily in the upper layer of the filter. This causes resistance to flow and water pressure below the bed decreases, causing an increase in the loss of head. As the resistance to flow increases, accumulated floc particles penetrate deeper into the bed, and head loss continues to increase until backwashing is required. The amount of head loss shortly after washing the filter varies with the type of media. The higher the rate of filtration and the finer the media, the higher the initial head loss. Higher losses of head can cause the media to become tightly packed, creating solids breakthrough and difficulty in backwashing. The multimedia gauge is an extremely important guide for proper operation. Check it periodically to ensure its accuracy.
Backwashing Filters
After a filter has been operating for some time, turbidity and suspended solids collect on the surface and in the filter media to the extent that flow of water through the bed is limited and a significant loss of hydraulic head results. At this point, solids can shear loose and break through into the effluent. When these conditions occur, it is necessary to backwash the accumulated solids from the filter. Backwashing is done by reversing the flow, forcing filtered water up through the gravel and sand. This loosens the filter media, agitating the media grains against each other and washing accumulated solids off the grain surfaces. The wash water flows to waste, carrying the solids with it. The flow rate of backwash water should be sufficient for cleaning the media but not so much that loss of media results. Expand the filter media at least 20 to 25 percent for good cleaning action, although a 40 percent expansion is best in many cases. Higher expansions risk washing out some filter media along with the accumulated solids. You can increase media cleaning by increasing interparticle abrasion, although the bulk of the cleaning action is due to the force of the rising backwash water. The amount of water required for sufficient backwashing is typically 1 to 5 percent of the total amount of water filtered, with an average of 2 percent. The duration of backwash required depends on the type and size of filter media, temperature of the water, type of solids being removed, and backwash flow rate.
Section IV
REVERSE OSMOSIS
PRINCIPLES
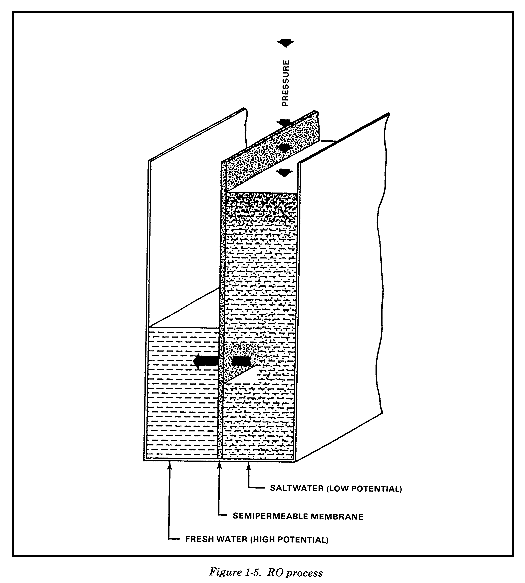
Reverse osmosis is a purification process in which filtered water is pumped against a semipermeable membrane under great pressure. The membrane allows product water to pass through while rejecting the impurities, both suspended and dissolved. You must use extremely high pressures to get a useful volume of water passing through a unit area of membrane. The reverse osmosis process is illustrated in Figure 1-5. Although RO can appear to be similar to a filtration process, there are distinct differences. In filtration, the entire liquid stream flows through the porous filter medium, and there are no changes in chemical potential between the feed and filtrate. In RO, the feed flows parallel to the semipermeable membrane with a fraction of it passing through a given membrane area; dissolved ionic and organic solutes are largely rejected by the membrane. RO removes selenium, copper, iron, manganese, chloride, lindane, radiation, and most color- and odor-causing compounds.
ELEMENT CHARACTERISTICS
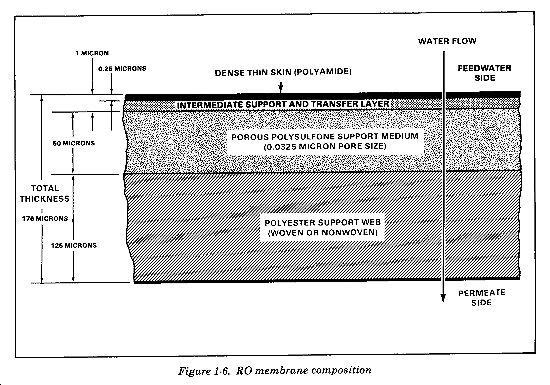
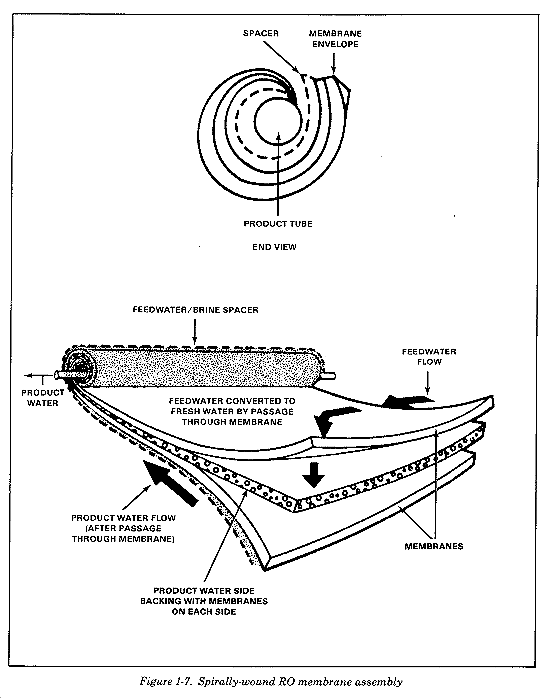
An RO element is composed of sheets of membranes (Figure 1-6) in a spirally-wound tube. Mesh spacers are inserted between layers of the membrane to allow water to flow into and out of the element. The meaning of spirally-wound is best understood if you review the partially unwound element in Figure 1-7. The center of the element is a plastic tube with small holes for the collection of product water. The leaves of membranes and spacers are rolled around the product water collection tube in the center of the element. The structure of the RO element allows water to flow from one end of the element to the other without any water passing through the membrane until the osmotic pressure is overcome. Water at lower than osmotic pressure flows through the elements and out the brine channel, but not into the product line. Under a lower than osmotic pressure condition, the elements, and consequently the membrane, are doing rather than through it, so water does not collect in the product water collection tube.
When the RO element is operating normally (at feed pressures in excess of the osmotic pressure), the concentrated brine (waste) stream flows out through the feedwater spaces. The brine collects at the end of the last element and flows out of the pressure vessel. Product water passes through the membrane into the product water channel from both sides. The product water entering the mesh from the membrane flows spirally towards the central product water collection tube. At the very center of the element, the product water channel butts up against the holes in the product water collection tube. Water passes from the product water channel into the product water collection tube, and then flows out of the pressure vessels and finally into the product water piping. Disinfect this water and store it as no work. The water just passes by the membrane potable product water.
Feedwater does not seep into the product water channel because three sides of the leaf of two membrane sheets and the product water channel mesh are glued together. Two of the glued sides become the ends of the element. This isolates the product water channel from feedwater on one end of the element and brine on the opposite end. The third side becomes a seam which stops feedwater from reaching the product water channel without passing through the membrane. The mesh must protrude from this membrane sandwich on the remaining side so that it can butt up against the product water collection tube. Because of this arrangement, only water that has passed through the membrane can enter the product water channel mesh.
The wagon wheel-shaped plastic stems which extend from the central product water collection tube to the outside perimeter of the element are called antitelescoping devices. These stems form a frame which prevents telescoping of the membrane. Telescoping describes the condition when the feedwater spacer begins to extend beyond the membrane leaves at the ends of the element. Excessively high pressure operation of the RO purifier could lead to telescoping if not for these devices.
MEMBRANE FOULING
Undesirable characteristics of the feedwater ahead of the RO system may occur. These undesirable characteristics are called fouling. Calcium carbonate precipitation may cause fouling of the membranes. You can reduce this fouling by lowering the pH of the water and by applying an inhibitor, such as sodium hexametaphosphate. This will prevent or reduce precipitation fouling of the membrane surface. Fouling of RO membranes may be caused by colloidal suspensions, dissolved salts, or chemical/physical interactions.
Colloidal Suspensions
Colloidal solids in the influent may be trapped in the membrane. These solids may interfere with either the flow through the element, reducing the quantity of product water, or affect the rejection characteristics of the membrane, thereby reducing the quality of the product water.
Dissolved Salts
Dissolved salts in the influent may be concentrated by the membrane so that their volubility product is exceeded. This may result in precipitation of these salts on or near the membrane.
Chemical/Physical Interactions
Chemical or physical reactions between certain feed components and the membrane may cause fouling. Potential foulants are either colloidal materials or dissolved materials. The most common colloidal foulants are iron, manganese, silica, very fine clay minerals, iron bacteria, organics (humic and fulvic acid-type compounds), bacteria, and algae. The most common dissolved foulants are calcium and aluminum.
FOULING PREVENTION
There are several different ways to minimize the problem of membrane fouling.
You can pretreat the influent stream to remove the fouling materials before they reach the membrane. This pretreatment may be by precipitation, filtering, or activated carbon.
You can pretreat the influent stream to dissolve the fouling materials. The most common example is the injection of citric acid to prevent the formation of calcium carbonate.
You can pretreat the influent stream to suspend the fouling materials. An example of this pretreatment is the injection of a dispersant such as sodium hexametaphosphate.
PRETREATMENT
In order to avoid fouling of the membrane surface, you must pretreat the feedwater. This is done by the multimedia filter, aided by a coagulant, and the cartridge filter (micron filtration). The desired turbidity of the membrane feedwater is 1 JTU or less. Pretreatment reduces the frequency of cleaning of the elements to an acceptable level and reduces the effect of fouling so that the RO system will continue to produce potable water. The objective of pretreatment is to reduce the effect of fouling so that continuous RO water purification operations can meet mission requirements. For additional information on pretreatment, see Sections II and III of this chapter.
Section V
DISINFECTION
DISINFECTANT CRITERIA
Water must be disinfected to be considered potable. No other treatment process, or combination of processes, will reliably remove all disease-producing organisms from water. All methods of disinfection must satisfy the following criteria. The disinfectant should--
- Mix uniformly to provide intimate contact with potentially present microbial populations.
- Have a wide range of effectiveness to account for the expected changes in the conditions of treatment or in the characteristics of the water being treated.
- Not be toxic to humans at the concentration levels present in the finished water.
- Have a residual action sufficient to protect the distribution systems from microbiological growths and act as an indicator of recontamination after initial disinfection.
- Be readily measurable in water in the concentrations expected to be effective for disinfection.
- Destroy virtually all microorganisms.
- Be practical to use and maintain.
DISINFECTION AGENTS
Chlorine, ozone, and chlorine dioxide are disinfection agents. They are described in detail below.
Chlorine is the disinfectant agent usually specified for military use. Presently, this is the only widely accepted agent that destroys organisms in water and leaves an easily detectable residual that serves as a tracer element. Sudden disappearance of chlorine residual signals potential contamination in the system. No other available disinfectant is as acceptable or adaptable for potable water treatment operations as chlorine. A major disadvantage is that chlorine reacts with certain organic compounds to form trihalomethanes, a known carcinogen.
Types of hypochlorite. There are two types (dry and liquid) of hypochlorite. These are explained below.
Dry. Dry hypochlorites added to water form hypochlorite solutions containing an excess of alkaline material, which tend to increase the pH. If the pH of the hypochlorite and water mixture rises high enough, calcium in the water and in calcium hypochlorite precipitates as calcium carbonate sludge. If this occurs, allow the hypochlorite and water mixture to stand so that the calcium carbonate may settle out. After the liquid hypochlorite solution settles, decant it into a separate tank for use. Two dry hypochlorites are calcium hypochlorite and lithium hypochlorite. HTH products contain about 70 percent available chlorine and 3 to 5 percent lime. Calcium hypochlorite is available in granular, powdered, or tablet forms and is readily soluble in water. Ship granular and tablet forms in 35- or 100-pound drums, cases, or smaller reusable cans. This is the form of chlorine found most often in Army water purification operations. Lithium hypochlorite contains about 35 percent available chlorine, readily dissolves in water, and does not raise the pH as much as other hypochlorite forms. Lithium hypochlorite is available in granular form. It is generally used for disinfecting swimming pools.
Liquid. The liquid solutions are clear, light yellow, strongly alkaline, and corrosive. They are shipped in plastic jugs, carboys, and rubber-lined drums of up to 50-gallon volumes. Sodium hypochlorite is available commercially in liquid form, such as Clorox. Household bleach is a sodium hypochlorite solution containing about 5 percent available chlorine. The usual concentration of sodium hypochlorite is between 5 and 15 percent available chlorine. Addition of sodium to drinking water may be hazardous to persons with hypertension or on low- or no-sodium diets.
Storage. High-test hypochlorites are relatively stable. Storage at temperatures below 86°F reduces the rate of deterioration. Unlike calcium hypochlorite, which can be stored for up to a year, sodium hypochlorite solution has a shelf life of only 60 to 90 days. Store sodium hypochlorite solutions in dry, cool, and darkened areas or in containers protected from light. Store hypochlorite solutions in rubber-lined or PVC-lined steel tanks fed through fiberglass, saran-lined, or PVC piping. Do not store or use dry hypochlorite in the presence of oil because of the fire potential.
Ozone
Ozone is an unstable form of oxygen that kills organisms faster than chlorine. Ozone, as a disinfection agent, is less influenced by pH and water temperature than chlorine. Another advantage of ozone is that it does not form compounds that create or intensify odors in the water. The main disadvantage of ozone is that it provides no lasting residual disinfecting action. Also, because of its instability, ozone is usually generated at the point of use. The process is not as adaptable to variations in flow rate and water quality as chlorine.
Chlorine Dioxide
Chlorine dioxide is a red-yellow gas or liquid with a very irritating odor. It has over 2 1/2 times the oxidation capacity of chlorine; but its rate of reaction is slower, and the mechanism of disinfection is completely different. It is effective over a broad pH range (5 to 9) and is not affected by sunlight. A major advantage of chlorine dioxide is that it does not hydrolyze in water and will not react with organic compounds to form trihalomethanes.
CHLORINATION EFFECTIVENESS
Use chlorination for disinfection of potable water in all cases with the exception of individual or small unit water purification for which you can use iodine tablets. The efficiency of chlorine disinfection is affected by the following variables.
A combination of the form of chlorine present, the pH of the water, and the contact time. As the pH of the water increases from 5 to 9, the form of the chlorine residual changes from hypochlorous acid to hypochlorite ion, which is less effective. The most effective disinfection occurs when the pH is between 5.5 and 6.5. At the same pH, a longer contact time also results in increased disinfection. Contact time is the time elapsing between the introduction of the chlorine and the use of the water. The required contact time is inversely proportional to residual, within normal limits. If the residual is halved, the required contact period is doubled. Army standards specify a minimum of 30 minutes contact time before product water is tested for residual to determine potability. A residual of 5 ppm must be maintained for water to be potable.
The type and density of organisms present (virus, bacteria, protozoa, helminth, or other) and their resistance to chlorine. Bacteria are most susceptible to chlorine disinfection whereas the cysts of the protozoa Entamoeba histolytica and Giardia lamblia are the most resistant.
The temperature of the water. At lower temperatures, the microorganism kill rate tends to be slower, and you need higher chlorine residuals or longer contact times. You obtain greater disinfection efficiency in warm water than in cold water. Longer contact time or increased chlorine dosages are required when the water temperature is low. Effectiveness of free chlorine at 35°F is about half of that at 70°F.
The concentration of substances other than disease-producing organisms that exert a chlorine demand. During disinfection, chlorine demand can be exerted by chemical compounds such as those containing ammonia and organic material. When these reactions occur, the chlorine is not available for disinfection. Chlorine demand is chlorine required to react with chlorine-destroying compounds. You must satisfy chlorine demand before disinfection can begin. Some of the compounds in water that exert a chlorine demand include iron, manganese, hydrogen sulfide, ammonia, and miscellaneous organic compounds. Add sufficient chlorine to the water supply to satisfy the chlorine demand, in addition to the amount required for actual disinfection.
Adequate mixing of chlorine and chlorine-demanding substances. Thoroughly mix the disinfecting agent to ensure that all disease-producing organisms come in contact with the chlorine for the required contact time.
The suspended solids concentration. Suspended solids can surround and protect organisms from the disinfectant.
CHLORINATION TREATMENT
Chlorination treatment consists of combined residual chlorination, free residual chlorination, and breakpoint chlorination. These treatments are described below.
Combined Residual Chlorination
Combined residual chlorination involves applying chlorine to water to produce a combined available chlorine residual and to maintain that residual through the water treatment and distribution operations. Combined available chlorine forms are less effective as disinfectants than free available chlorine forms. You need about 25 times as much combined available residual chlorine to obtain equivalent bacterial kills as required for free available residual chlorine under the same conditions of pH, temperature, and contact time. You need about 100 times longer contact time to obtain bacterial kills for equal amounts of combined versus free available chlorine residuals under similar conditions. You can use combined residual chlorination to control algae and bacterial aftergrowth in potable water distribution systems. Combined chlorine residuals can maintain a stable residual throughout the system to the point of usage at the distribution point. In some cases, you can use free residual chlorination to ensure effective disinfection, followed by the addition of ammonia to convert the free residual to a combined available residual.
Free Residual Chlorination
Free residual chlorination involves producing a free available chlorine residual through part or all of the water treatment and distribution operations. You can form free available chlorine residuals by applying chlorine to water, if the water contains no ammonia or other nitrogenous materials. If the water contains ammonia and combined available chlorine residuals are formed, add sufficient chlorine to destroy the combined chlorine residual. Free residual chlorination provides initial disinfection with a contact period of about 10 minutes, whereas combined chlorine residual requires at least 60 minutes. Changes in pH and temperature do not markedly affect the disinfecting powers of the free chlorine residual. A combined chlorine residual must be increased significantly with increases in pH and decreases in temperature. You can diminish taste and odors by using free residual.
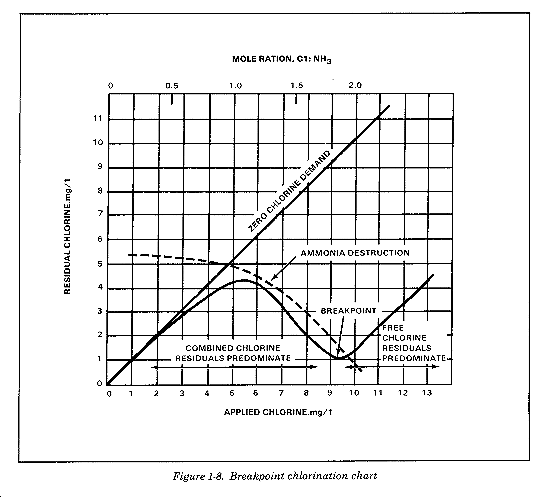
Breakpoint Chlorination
Breakpoint chlorination is the application of chlorine to produce a residual of free available chlorine with minimum combined chlorine present. Adding chlorine to water with ammonia forms chloramines. With additional application, chlorine residuals increase and reach a maximum when the ratio of chlorine to ammonia is equal. As you apply greater dosages of chlorine and the ratio of chlorine to ammonia increases, you oxidize ammonia by the chlorine and reduce the chlorine residual. When you add approximately 10mg/L of chlorine for each mg/L of ammonia present, chloramine residuals decline to a minimum value. This is the breakpoint and represents a point where further addition of chlorine produces a free residual (Figure 1-8). The actual amount of chlorine required to arrive at the breakpoint varies between 7 and 15 times the ammonia nitrogen content of the water. Due to the presence of organic and other chlorine reactive materials, however, you may need 25 times as much chlorine as ammonia nitrogen content to reach breakpoint. Beyond breakpoint, the residual should have at least 90 percent free available residual chlorine. The rate of the breakpoint reaction appears to be most rapid between a pH of 7 and 8, and it increases with a higher temperature. At pH levels below 8, you can form nitrogen trichloride following breakpoint treatment and impart odors to the water. You must expose the water to air to provide for the release of nitrogen trichloride.
|
NEWSLETTER
|
| Join the GlobalSecurity.org mailing list |
|
|
|