U.S. Policy Regarding Pandemic-Influenza Vaccines
Chapter
1
The Market for Seasonal-Influenza Vaccine and the Challenge of Providing Vaccine in a Pandemic
The prospect of an influenza pandemic—a global outbreak of influenza that leads to serious illness or death in large numbers of people—is cause for concern among policymakers, public health experts, and the public at large. Pandemics are not new: There were three in the 20th century, the deadliest of which, the "Spanish flu" of 1918, caused global devastation, killing more than 500,000 people in the United States and about 50 million people worldwide. According to the World Health Organization (WHO), an influenza pandemic requires three conditions: First, a new virus emerges to which people have little natural immunity. Second, infection leads to significant rates of illness or death. And third, the virus is transmitted efficiently from one person to another (WHO 2006, p. 1).
Although a pandemic could be caused by any of several influenza strains, policymakers and public health experts are particularly worried about the persistence of the currently circulating H5N1 strain (the "avian flu" or "bird flu"), which has caused high mortality among poultry in Asia and has spread in poultry from Southeast Asia to Central Asia, Europe, and Africa.1 The H5N1 virus meets two of the three conditions for a pandemic: First, people have little natural immunity to H5N1 because it has not widely circulated among the human population. And second, it has caused significant illness in the 385 people who have become infected, and 243 of those people have died. The mortality rate from the H5N1 virus is thus in excess of 60 percent of known cases (WHO 2007a).2 So far, WHO’s third condition has not been met: The H5N1 virus is not transmitted efficiently from one person to another. Close contact with infected poultry is thought to be required for human infection. However, the danger exists that the virus will evolve in a way that allows for efficient human-to-human transmission, perhaps leading to a pandemic. Depending on the virulence of the particular strain of influenza, a pandemic could have substantial consequences for human health and for economic activity around the world (CBO 2005, 2006a). Because infectious diseases are unpredictable, public health authorities cannot say for sure when a pandemic will arise, whether it will involve H5N1 or some other strain, or whether it will be mild or virulent.
Against the prospect of a pandemic like the 1918 Spanish flu, the Department of Health and Human Services (HHS) since 2004 has budgeted about $7.9 billion for activities to support a research and preparedness plan for an influenza pandemic (see Table 1-1). A large portion of that amount—nearly $5.6 billion—came in supplemental appropriations for 2006 to fund HHS’s plan for coping with an influenza pandemic (HHS 2005b).3 The department’s national response plan includes support for research and development in new vaccines and new vaccine formulations, an increase in production capacity, and the establishment of vaccine stockpiles. The plan also encompasses the stockpiling of existing antiviral drugs as well as other medical supplies (including surgical masks, respirators, ventilators, and syringes) and the development of new antiviral drugs. In addition, HHS’s recommendations address the coordination of state and local preparedness and response and methods for monitoring influenza viruses that have the potential to cause a pandemic.
HHS’s Funding for Influenza Preparedness, 2004 to 2008
(Budget authority, in millions of dollars)
2004 |
2005 |
2006 |
2006a |
2007 |
2008 |
Total,
2004-
2008 |
|||||||||
Office of the Secretary |
43 |
101 |
4 |
5,152 |
0 |
76 |
5,377 |
||||||||
Centers for Disease Control and Prevention |
198 |
323 |
295 |
400 |
73 |
73 |
1,361 |
||||||||
Food and Drug Administration |
3 |
5 |
5 |
20 |
33 |
38 |
103 |
||||||||
National Institutes of Health |
113 |
164 |
207 |
18 |
271 |
271 |
1,044 |
||||||||
Total |
357 |
592 |
511 |
5,590 |
377 |
458 |
7,885 |
||||||||
Source: Congressional Budget Office based on data collected from HHS.
Note: HHS = Department of Health and Human Services.
a. Additional funds were provided in the form of emergency supplemental appropriations.
HHS has budgeted $3.2 billion of its 2006 supplemental appropriations (plus some of its appropriated funds from other years) for vaccines (see Table 1-2). Relying mainly on the supplemental funding provided in 2006, HHS has obligated almost $2.6 billion to promote the development of vaccines, increase the investment in new production capacity, and procure vaccine stockpiles (see Table 1-3). According to agency officials, HHS has yet to obligate about $1.3 billion of the supplemental appropriations provided in 2006. About $900 million of that amount is budgeted for vaccine-related activities. Officials at HHS note that although those funds have not yet been obligated, there are plans for their use.
Supplemental Funding for HHS’s Pandemic-Influenza Plan, 2006
Budget Authority |
|||||
Millions of dollars |
Percentage |
||||
Vaccines |
3,233 |
58 |
|||
Antiviral Drugs |
911 |
16 |
|||
Medical Supplies |
170 |
3 |
|||
State and Local Preparednessa |
770 |
14 |
|||
Monitoringb |
455 |
8 |
|||
Other |
51 |
1 |
|||
Total |
5,590 |
100 |
|||
Source: Congressional Budget Office based on data from HHS (2006a).
Note: HHS = Department of Health and Human Services.
a. Includes funding for state subsidies for antiviral drugs.
b. Includes international and domestic activities.
HHS’s Obligations for Pandemic-Influenza Vaccine Projects
Obligationsa (Millions of dollars) |
Duration |
|||
Develop New Vaccines |
1,435 |
2005 to 2012 |
||
Increase Capacity |
176 |
2004 to 2012 |
||
Stockpile Vaccine |
950 |
2004 to 2008 |
||
Total |
2,561 |
|||
Source: Congressional Budget Office based on data from Robinson (2008).
Note: HHS = Department of Health and Human Services.
The President’s budgetary proposals for 2009 include $820 million for HHS to pursue its pandemic-influenza activities. Of that amount, $467 million is to procure vaccines for the stockpile and to fund vaccine production capability, $40 million is to stockpile other medical supplies, and $313 million is to fund preparedness activities at the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), the National Institutes of Health (NIH), and the Office of the Secretary in HHS.
The vaccine component of HHS’s plan is motivated by concerns about the capacity and capabilities of the current group of manufacturers of seasonal-influenza vaccine to respond to the threat of a pandemic. Because current vaccines and manufacturing capacity are inadequate to protect the U.S. population in the event of a pandemic, HHS is devoting much of its effort to developing new vaccines and expanding existing manufacturing capacity. Specifically, the first goal is to have in place by 2011 domestic production capacity sufficient to supply vaccine to the entire U.S. population within six months of the onset of a pandemic. The second goal is to stockpile enough doses of prepandemic vaccines to inoculate 20 million people. (Prepandemic vaccines are developed from strains that public health officials believe have the most potential to cause an influenza pandemic.) First priority for vaccination will be given to children and to people who are critical to maintaining security, health care, and essential services.
This Congressional Budget Office (CBO) paper examines several questions:
¦ |
What steps have been taken and what is the status of HHS’s plan to improve manufacturers’ response to an influenza pandemic? |
¦ |
What changes have occurred in the vaccine industry, particularly as a result of expanded government involvement in the influenza vaccine market? |
¦ |
What is the continuing role for the government in developing and producing influenza vaccines to meet the performance objectives specified under current policy, and how will that role affect federal spending? |
The Market for Seasonal-Influenza Vaccine
The first line of defense against seasonal influenza, which results in the hospitalization of about 200,000 people and causes about 36,000 deaths each year in the United States, is annual vaccination.4 About 100 million U.S. residents were inoculated in the 2006–2007 season. Vaccination is considered a principal strategy to combat pandemic influenza as well, but a pandemic will create huge surges in demand. The nation’s response to an influenza pandemic will depend to a great extent on whether the manufacturers of seasonal-influenza vaccine can meet the challenge of providing a far larger number of doses of the correct vaccine quickly enough to inoculate the whole population.
As it is currently formulated, the seasonal-influenza vaccine contains 15 micrograms (a microgram is one one-millionth of a gram) of active ingredient, or antigen (the protein that elicits an immune response in the body) from each of three strains of influenza virus, for a total of 45 micrograms per dose. (By contrast, a pandemic vaccine would contain antigen from a single strain.) Since the 1940s, manufacturers have made vaccines by injecting seed viruses into hens’ eggs, growing them there, and then using the viruses grown in the fluids inside the eggs as the starter for the vaccine.
The FDA has licensed two main types of vaccine: One, called a subunit vaccine, uses purified proteins from killed viruses. The subunit vaccine is delivered by injection and accounts for 97 percent of the vaccine used in the United States. The other type uses a weakened live form of the influenza virus (often called live, attenuated virus). The vaccine made from the weakened live virus is administered in an intranasal spray, and just one company produces it for the U.S. market.
Because the genetic makeup of influenza viruses can change rapidly, the vaccine must be reformulated each year to confer immunity against the strains researchers believe will circulate that year. In February or March, the FDA announces the strains, which are chosen on the basis of surveillance data from CDC and WHO. Manufacturers then produce vaccine for delivery between November and March, the peak influenza season in the Northern Hemisphere. It takes about six months from the time the virus strains are isolated for the process to play out: manufacturing, purification, testing, filling, packaging, and delivery to clinics and physicians’ offices.
The production of seasonal-influenza vaccine is characterized by high fixed costs—costs that do not change whether a manufacturer produces one dose of vaccine or millions. The most widely cited analysis puts the fixed cost of producing any vaccine at 60 percent of the total costs (exclusive of research and development costs), regardless of volume (Mercer Management Consulting 2002). Among the fixed expenses are depreciation, administration, quality assurance, and personnel. Another 25 percent of the cost is fixed at the batch level regardless of batch size. Only 15 percent of the cost is truly variable, fluctuating directly with the number of doses produced.
The proportion of fixed costs suggests that the manufacturers with the largest market share will enjoy lower average costs—a condition that is conducive to market concentration among vaccine producers. As the number of producers declines, the potential for supply disruptions caused by contamination or other problems rises.
For the 2006–2007 season, there were only four manufacturers of influenza vaccine for the United States: GlaxoSmithKline (GSK), Sanofi Pasteur, Novartis, and MedImmune (see Figure 1-1). And only one of those, Sanofi Pasteur, actually produces the vaccine domestically. In all, those manufacturers produced about 120 million doses of vaccine for the 2006–2007 season, of which about 100 million were distributed (see Figure 1-2). (Because seasonal-influenza vaccine is reformulated each season, manufacturers must discard undistributed vaccine every year.)
Vaccine Production for the 2006–2007 Influenza Season in the United States
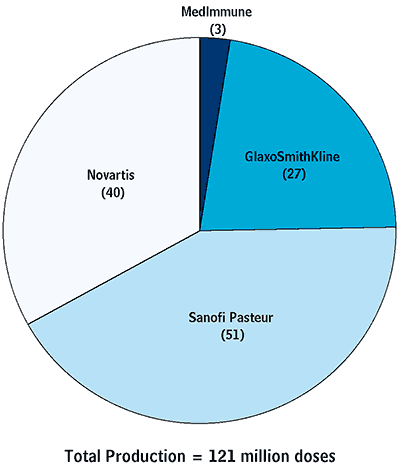
Source: Congressional Budget Office based on Health Industry Distributors Association (2007) and Novartis (2007).
Seasonal-Influenza Vaccine for the U.S. Market
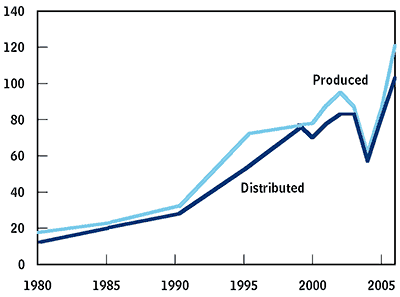
Source: Congressional Budget Office based on data from the Centers for Disease Control and Prevention.
The supply of seasonal-influenza vaccines has been disrupted several times in the recent past. In 2000–2001, manufacturers had difficulty growing one of the three influenza strains, and some facilities were shut down because of the FDA’s concerns about compliance with good manufacturing practices (Danzon, Sousa Pereira, and Tejwani 2005; Government Accountability Office 2007). In the 2003–2004 season, Wyeth’s decision to exit the market rather than incur the cost of upgrading its facility left just two manufacturers of injectable influenza vaccine. One of the two was unable to deliver any vaccine to the U.S. market for the 2004–2005 season because of contamination at its facility in the United Kingdom.
Between 1999 and 2006, list prices (which are set by manufacturers and reported to CDC) for influenza vaccine jumped from about $2 to about $11 per dose. In general, individual clinicians, clinics, and hospitals pay list price or close to it; large groups, state consortia, and health plans often can negotiate for rebates and discounts (Institute of Medicine 2004, pp. 127–128). The number of doses distributed rose from 77 million in 1999 to 100 million in 2006 (see Figure 1-2).
WHO has estimated that, in 2007, manufacturers produced 565 million doses for the global market (WHO 2007d). That quantity would still fall well short of the number needed to inoculate the world’s 6.6 billion people; by 2010, new production capacity could raise the global supply to 1 billion doses of seasonal-influenza vaccine.5
Supplying Vaccine in a Pandemic
If an influenza pandemic were to occur today, it would be impossible to meet HHS’s goal of vaccinating the entire U.S. population of about 300 million people within the next six months. To begin with, current capacity for domestic production would be completely inadequate. (Only 50 million of the approximately 120 million doses produced for the domestic market during the 2006–2007 influenza season were manufactured in the United States.) In the event of a pandemic, moreover, it could be difficult to obtain supplies from overseas, especially if there are shortages in vaccine-producing countries or if those countries vaccinate their own populations before permitting exports to the United States (Osterholm and Branswell 2005).
It also is anticipated that the pandemic-influenza vaccine would have to be stronger than the seasonal version. Because of the lack of previous exposure to the H5N1 virus, humans would be expected to have no immunity at all. The only H5N1 vaccine currently approved for the U.S. market requires a course of two doses at 90 micrograms per dose (FDA 2007). (The seasonal vaccine is administered in a single dose of 45 micrograms of antigen—15 micrograms against each of three strains. Most adults are exposed regularly to seasonal-influenza viruses and thus have some immunity, so the seasonal vaccine is in effect a booster shot. The course for children, who generally have less exposure and hence less immunity, typically is two doses.)
When all of those factors—uncertain availability of imports, higher content per dose, and more doses per course—are taken into consideration, current U.S. capacity to produce pandemic vaccine is only about 12.5 million courses (see Table 1-4). That estimate could be high; in the past, manufacturers have had difficulty growing pandemic-influenza strains. Moreover, given the six months it takes to produce and distribute current vaccines, experts fear that the pandemic-influenza vaccine would not be available until after the first wave of a pandemic had passed.
Domestic Production Capacity for Seasonal- and Pandemic-Influenza Vaccine
Adjustment Factors |
Assumptions |
|||||||
Seasonal-Influenza Vaccine |
50 Million Doses |
|||||||
Adjustments for |
||||||||
Strains per dose of pandemic vaccine |
× 3 |
One strain instead of three |
||||||
Antigen per dose of pandemic vaccinea |
÷ 6 |
90 micrograms instead of 15 micrograms against a single strainb |
||||||
Doses per course of pandemic vaccine |
÷ 2 |
Two doses per coursec |
||||||
Pandemic Vaccine for U.S. Market |
12.5 Million Courses |
|||||||
Source: Congressional Budget Office.
a. Antigen is the active ingredient in a vaccine.
b. A microgram is one one-millionth of a gram.
c. For seasonal vaccines, a course generally consists of a single dose. Researchers believe the pandemic-influenza vaccine will require a course consisting of two doses.
An influenza pandemic therefore would present a significant challenge for public health authorities and for manufacturers. About one-third of the U.S. population was vaccinated in the 2006–2007 season. If a pandemic were to occur, demand for vaccine would be much greater, even if vaccinating the entire U.S. population were not a policy goal. It would be a significant undertaking to increase output by so much, so quickly.
Because domestic vaccine manufacturers right now could not quickly provide enough vaccine to meet the threat of an influenza pandemic, HHS has focused on increasing domestic capacity for production. In the future, the agency plans to focus on reducing production time.
The emergence of H5N1 as a human health risk found a U.S. manufacturing base reduced to a single domestic manufacturer, producing the vaccine in an egg-based process developed in the 1940s. The first step in the HHS plan was to promote an increase in capacity as rapidly as possible by encouraging the expansion and refurbishing of existing plants. The second, and current, step is to introduce cell-based manufacturing technology to the domestic production of vaccine. Cell-based production uses animal cells to culture the virus, and it is a standard method for producing other vaccines, including several against childhood diseases.
It would be more expensive and time-consuming to initiate cell-based manufacturing than it would be to add capacity for egg-based production. But, HHS contends, adding cell-based capacity reduces the risk that is inherent in egg-based technology—that the laying hens could become infected with H5N1. HHS also argues that it allows for the possibility of producing the quantities of vaccine that would be needed in a pandemic.
HHS’s plan includes short- and longer-term approaches to solving the problem of how to make the vaccine available quickly in the event of an influenza pandemic. In the short run, the procuring of a limited stockpile of vaccines would permit the government to expedite an initial response. But because stockpiled vaccines are made before a pandemic virus emerges, the formulations are not expected to perfectly match the pandemic virus, and they might not provide adequate protection. Moreover, the size of the current stockpile would permit inoculation of only a limited number of people.
HHS’s long-term plans to promote the development of the next generation of vaccines—perhaps taking advantage of new methods in biotechnology—would address the problems of capacity shortage and of long production times. However, because developing safe and effective vaccines could take years, perhaps a decade or more, HHS’s plan would encourage pharmaceutical manufacturers to start development now so that they are more likely to be able to produce vaccines quickly if a pandemic occurs in the future.
In parallel with those efforts, HHS is funding the development of adjuvants, substances that could boost the potency of the antigen in vaccines, thus reducing the amount of active ingredient needed per dose. Their successful development could affect many areas of HHS’s plan. The use of adjuvants for egg-based and cell-based vaccines could allow for the production of more doses of vaccine from existing facilities, and fewer new manufacturing facilities would need to be built. Moreover, smaller stockpiles could be set aside to protect larger numbers of people.
Risks Associated with HHS’s Plan
Any program that targets a problem as complex as an influenza pandemic entails risks and costs. The technology for cell production, for example, has been expensive to develop, and building the necessary facilities will require yet more resources. Yet, should technology develop successfully for the next generation of vaccines, the newly refurbished production facilities for egg-based products and the newly constructed facilities for cell-based vaccines would no longer be needed for producing influenza vaccine. The egg-based production facilities could become obsolete and perhaps be discarded while the facilities for cell-based production could be put to other uses.
A strategy of building up egg-based capacity while focusing on next-generation technology could avert some of the expense associated with modernizing the manufacturing technology. The trade-off would come with the possible exposure of the country to risk if the development of next-generation technology vaccines were delayed or never occurred at all.
Governments abroad have chosen different strategies in the face of the possibility of an influenza pandemic. Several member states of the European Union have entered into advance supply agreements with vaccine manufacturers. The governments agree to pay the manufacturers in advance, and the vaccine makers guarantee they will provide a specified number of doses of vaccine within a set period, such as six months. The governments’ expectation is that the manufacturers (or the U.S. government) will develop the requisite vaccines and manufacturing technology. Other governments have given much less support for technology development than has the U.S. government. Given that seasonal-influenza vaccine is largely privately purchased in the United States, some analysts have asked whether substantial federal support for technology development is appropriate, especially compared with other governments’ investments.
Although the threat of a pandemic on the scale of the Spanish flu of 1918 looms large in public health calculations, public health officials also recall the prospect in 1976 of an outbreak of what was known as the swine flu. In that instance, there was a widely perceived threat, and a federal vaccine program was initiated rapidly (Allen 2007, p. 261; Kolata 1999, pp. 121–185). The government determined it would inoculate 200 million U.S. residents within six months. In the end, some 40 million people received the vaccine, double the number ever vaccinated in a single year. However, the pandemic never appeared, and within months the vaccine became associated in public opinion—possibly incorrectly—with Guillain-Barré syndrome, a rare neurological disorder. The federal government ended up paying $100 million in compensation. Because of that history of premature response and backlash, and because vaccines are drugs that are given to healthy people, the public health community is cautious about introducing new vaccines into the market even in anticipation of a global health threat.
Additional Public Health Questions
The government’s role in the vaccine market under HHS’s plan also raises important questions for the medical and public health communities about whether the plan provides adequate protection against the threat of a pandemic. For example, HHS will need to ensure that it has mechanisms in place to identify the recipients and distribute the vaccines from the stockpile and from manufacturers as the vaccines are being produced. Policymakers must decide who will pay for pandemic vaccines. Currently, most influenza vaccine is purchased by the private sector. Would the same conditions obtain during a pandemic? Those issues are beyond the scope of this paper, which focuses on the development of new vaccines and the capacity to manufacture them.
Influenza viruses that affect humans, birds, and other animals are named for two surface proteins, hemagglutinin and neuraminidase. The surface of H5N1, accordingly, has one type 5 (of the possible 16) hemagglutinin protein and one type 1 (of 9) neuraminidase protein (CDC 2005).
The mortality rate, however, might in fact be substantially lower. Public health authorities do not know how many people with milder cases did not seek medical care or how many received care that was not reported.
A supplemental appropriation is an act of Congress appropriating funds in addition to those already contained in the usual annual appropriation legislation.
According to CDC, "Influenza is a respiratory illness. Symptoms of flu include fever, headache, extreme tiredness, dry cough, sore throat, runny or stuffy nose, and muscle aches. Children can have additional gastrointestinal symptoms, such as nausea, vomiting, and diarrhea, but these symptoms are uncommon in adults. Although the term 'stomach flu’ is sometimes used to describe vomiting, nausea, or diarrhea, these illnesses are caused by certain other viruses, bacteria, or possibly parasites, and are rarely related to influenza" (CDC 2008).
Recommendations for meeting potential global demand for pandemic vaccine are discussed by David Fedson (2003).
|
NEWSLETTER
|
| Join the GlobalSecurity.org mailing list |
|
|
|




