
Taiwan's ACIP approves Moderna vaccine for children aged 6-11
ROC Central News Agency
04/20/2022 09:40 PM
Taipei, April 20 (CNA) Taiwan's Advisory Committee on Immunization Practices (ACIP) approved the use of the Moderna COVID-19 vaccine for children aged 6-11 Wednesday, as well as third doses for the 12-17 age group and fourth doses for certain high-risk groups.
The Moderna vaccine is the first in Taiwan to be approved for children under 12 years old.
Under the ACIP's guidelines, two 0.25 milliliter doses of the vaccine, each containing 50 micrograms of mRNA -- half of the adult dosage -- will be administered to the 6-11 age group with a minimum interval of 12 weeks, the Central Epidemic Command Center (CECC) said in a statement.
The CECC will arrange for children to receive the vaccine in schools, as was the case with 12-17-year-olds, and they will also have the option to get the vaccine in hospitals.
The rollout is expected to begin in early May, CECC spokesperson Chuang Jen-hsiang (莊人祥) told reporters.
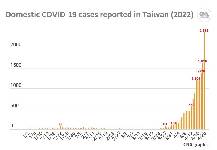
The figures do not include imported cases reclassified as domestic ones, nor retroactively removed cases. As of April 20, Taiwan recorded 15,544 domestic cases in 2022, while the total number of imported cases rose to 9,985 from 2,375 on Dec. 31.
Taiwan's Food and Drug Administration (FDA) authorized the use of Moderna's COVID-19 vaccine in children aged 6-11 on Sunday, after determining that the vaccine met the necessary safety and efficacy requirements.
The ACIP also decided Wednesday to allow those in the 12-17 age group to get a booster shot at least five months after receiving their second dose, the CECC statement said.
The 12-17 age group has been approved to get the Pfizer-BioNTech vaccine and the Moderna vaccine, though Taiwan has run out of the former at present, according to the CECC.
As this age group only began to receive their second shot in mid-December, the rollout of booster doses will begin in mid-May at the earliest, Chuang said.
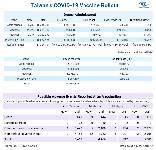
1. More doses of the Moderna and Pfizer-BNT vaccine have been administered in Taiwan than the government has officially received because recipients of the Moderna booster shot are given half the standard dose of the first and second jab, while medical workers can sometimes get more than the standard number of shots from a Pfizer-BNT vial. 2. Information about the booster dose and additional dose can be found at https://t.ly/4ZuW
Also on Wednesday, the ACIP approved giving a fourth dose of a COVID-19 vaccine to seniors aged 65 and above, as well as for residents of long-term care facilities.
Individuals aged 18 and above who are immunocompromised have also been given the approval to receive a fifth vaccine dose. This group was previously approved to get an additional dose due to their weakened immune systems and a booster dose, the CECC said.
These include cancer patients who have received treatment to suppress their immune system in the past year; organ or stem cell transplant recipients; people with moderate or severe primary immunodeficiency; dialysis patients; people with HIV; people taking medications that weaken the immune systems; and people who have received chemotherapy or radiation therapy in the past six months.
Those receiving their fourth or fifth vaccine dose should get it at least five months after their last shot, and they can choose between the Moderna, Pfizer-BioNTech, Medigen, or Novavax brands, the CECC said.
Taiwan has not yet received any doses of the Novavax vaccine, though the CECC announced in March that the country had ordered around 2 million doses of the Novavax COVID-19 vaccine through the global vaccine sharing initiative COVAX.
To date, 84.24 percent of Taiwan's population has gotten at least one dose, 79.49 percent has gotten two doses, and 55.99 percent has received a third dose, CECC data shows.
(By Chiang Yi-ching)
Enditem/ASG
|
NEWSLETTER
|
| Join the GlobalSecurity.org mailing list |
|
|
|

